40 lewis diagram for n2
Marshall County is a county located on the south central border of Oklahoma. As of the 2010 census, the population was 15,840.[1] Its county seat is Madill.[2] How to Draw the Lewis Structure of N2 - with explanation!Check me out: http://www.chemistnate.com
Lewis diagram for the ether c2h5oc2h5
Lewis diagram for n2
A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t... Nitrogen sinks in some knee structure or nearby structure could be physical, chemical, or physiological. The authors conclude that these unexpected results of a very marked delay in knee gas excretion 30 minutes into the pulmonary washout period suggests that a gas exchange model consistent with these data is needed to avoid decompression sickness. Based on the Lewis electron-dot diagrams of N2 and N2H4, N2 has a stronger nitrogen-to-nitrogen bond than N2H4.. The strength of a bond is dependent on the bond length and the bond order.The higher the bond order, the shorter and stronger the bond.Hence triple bonds are stronger than double bonds and double bonds are stronger than single bonds.. Having said that, N2H4 contains single bonds ...
Lewis diagram for n2. 11+ N2 Lewis Structure. The lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs ) and one shared pair of electrons (written between the atoms). Calculate the total valence electrons in no2 molecule. Nitrogen Fixing Plants to Fertilize… A Lewis Dot Structure is drawn by a series of dots, lines, and atomic symbols and provides a structure for the way that the atom or molecule is arranged. A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures The Lewis Structure for N2 looks easy at first. The problem is that there aren't enough valence electons to give both Nitrogen atoms an octet. You'll need to use . The Lewis dot structure for any molecule can be found by following a general set of This is derived by following 5 general steps for writing Lewis dot structures. In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. Here is the electron dot structure for a single N atom: The total number of valence electrons between the two N atoms is 10 e^-.
NO2 (Nitrogen Dioxide) Lewis Dot Structure. Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3. Answer (1 of 3): N2 Lewis Structure Answer: In N2 Lewis structure,two nitrogen atoms has shared six valence electrons and every nitrogen atom has one lone pairs .In N2 Lewis structure,there are ten valence electrons. N2 Lewis Structure Explanation: Hello,today I am going to draw the N2 Lewis... Nov 12, 2021 · NH2 Lewis Structure. The most initial and important step towards finding out the bonding nature of any chemical molecular structure is to draw its Lewis Structure. Lewis Structure is a 2D diagram to represent the internal bonding between constituent atoms in a molecule or ion. Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
Cl2 Br2 H2 O2 N2 HCl DOUBLE bond atoms that share two e- pairs (4 e-) O O TRIPLE bond atoms that share three e- pairs (6 e-) N N Draw Lewis Dot Structures You may represent valence electrons from different atoms with the following symbols x, , CO2 NH3 Draw the Lewis Dot Diagram for polyatomic ions Count all valence e- needed for covalent ... which of the following complete lewis diagrams represents a molecule with a bond angle that is closest to 120º? ... the particle-level diagram represents the structure of solid KF. although the molar mass of KCl is greater than that of KF, the density of KCl is actually less than that if KF. why? ... N2. the energy required to dissociate an ... A Lewis diagram shows two nitrogen atoms double-bonded toge... View Answer. Below is the Lewis structure of the nitrogen \left ( N_{2} \right ) molecule.Count the number of bonding pairs and the ... Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
In N2 Lewis structure,two nitrogen atoms has shared six valence electrons and every nitrogen atom has one lone pairs .In N2 Lewis structure,there are ten ...3 answers · 4 votes: As nitrogen is in fifth group in periodic table therefore it will have five electrons in the ...
Nitrogen (N 2) Molecule Lewis Structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure.. N 2 lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.
Transcript: For the N2 Lewis structure, we have five valence electrons for Nitrogen--it's in group 5 or 15 on the periodic table. We have two Nitrogens. Multiply those together, we have a total of 10 valence electrons for the N2 Lewis structure. We'll put the two Nitrogens next to each other, and then we'll put two valence electrons between them to form a chemical bond.
N2 Lewis Structure In the N2 molecule, there are only two atoms are present. According to the periodic table, each n2 nitrogen comes with the 5 valence electrons. The nitrogen molecule contains 2 nitrogen atoms so a total of 10 valence electrons in the n2 molecule. The first step in the Lewis diagram is
1 answerThe given formula corresponds to a simple diatomic covalent molecule of nitrogen. Nitrogen (N) itself contains 5 valence electrons as a main group 5A...
N2 Lewis Structure The N2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair. N2 Lewis Structure Setup It's easiest to think in terms of […]
Drawing the Lewis Structure for N 2 (Dinitogen or Nitrogen Gas). Nitrogen (N 2) is a commonly tested Lewis structure due to its importance on Earth (about 78% of the Earth's atomsphere is N 2).It also is a good example of a molecule with a triple bond. There are 10 valence electrons available for the Lewis structure for N 2.. Video: Drawing the Lewis Structure for N 2
Nov 13, 2021 · Lewis diagram is a pictorial presentation of the number of valence electrons present in an atom, which readily reacts with the valence electrons of another atom to form a bond. The diagram is drawn with the help of eight dots around the atom, mostly in pairs. Here, the number eight has been selected as per the octet rule.
Lewis Structure for NO 2-(Nitrite ion). Lewis structure of NO 2-ion is drawn in this tutorial. Total valence electrons of nitrogen and oxygen atoms and negative charge also should be considered in the drawing of NO 2-lewis structure.. Now, we are going to learn, how to draw this lewis structure.
diagram B 14 Solution In B2, C2, and N2, the 𝜎2𝑝 orbital is higher in energy than the 𝜋2𝑝 orbitals as shown in diagram A. In O2 and F2, the 𝜋2𝑝 orbitals are higher in energy than the 𝜎2𝑝 orbital as shown in diagram B. The number of valence electrons per atom of an element is related to …
What is the Lewis dot diagram for nitrogen? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable. The two letter N's in the N2 Lewis structure represent the nuclei (centers) of the ...
Considering the energy level diagram, the configuration of N2 is σ1S2, σ *1S2, σ2S2, σ*2S2, π2Px2, π2Py2, σ2Pz1. Conclusion. In the Lewis structure of the N2 molecule, there is a formation of a triple covalent bond represented by three lines between two atoms of Nitrogen.
Lewis Structure Of N2- Key Points. In the lewis structure of N2, there is a triple bond between two nitrogen atoms. The molecular geometry of N 2 is linear. N2 is colorless, odorless, and tasteless gas. Number of electrons in the valence shell of nitrogen atom = 5; Each nitrogen atom is surrounded by a lone pair of electrons. Hybridization of ...
SOLVED N2 Lewis Structure(Nitrogen), Is Nitrogen(N2) Polar or Nonpolar? Molecular Geometry and Bond Angle of Nitrogen - Solution Platform
Lewis Structure Let's look at the Lewis structure of and N 2. Atomic nitrogen has 5 valence electrons and 4 valence orbitals (2s, 2p x, 2p y, and 2p z). In the Lewis structure there is a triple bond between the nitrogen atoms and a non-bonding pair of electrons on each.
Lewis structure of N2. A Lewis Structure is a very simple representation of the valence, or outermost, electrons in a molecule. It does not explain the geometry of the molecule, but it is a step forward in approaching the geography. But to find out if N2 is polar or nonpolar, the Lewis Structure can reveal the best electron makeup of the molecule.
Lewis dot diagram for n2. Drawing the lewis structure for n 2 dinitogen or nitrogen gas nitrogen n 2 is a commonly tested lewis structure due to its importance on earth about 78 of the earths atomsphere is n 2. A step by step explanation of how to draw the n2 lewis dot structure nitrogen gas.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
Based on the Lewis electron-dot diagrams of N2 and N2H4, N2 has a stronger nitrogen-to-nitrogen bond than N2H4.. The strength of a bond is dependent on the bond length and the bond order.The higher the bond order, the shorter and stronger the bond.Hence triple bonds are stronger than double bonds and double bonds are stronger than single bonds.. Having said that, N2H4 contains single bonds ...
Nitrogen sinks in some knee structure or nearby structure could be physical, chemical, or physiological. The authors conclude that these unexpected results of a very marked delay in knee gas excretion 30 minutes into the pulmonary washout period suggests that a gas exchange model consistent with these data is needed to avoid decompression sickness.
A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t...













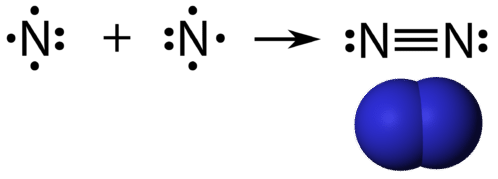















0 Response to "40 lewis diagram for n2"
Post a Comment