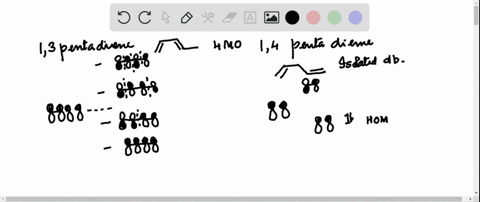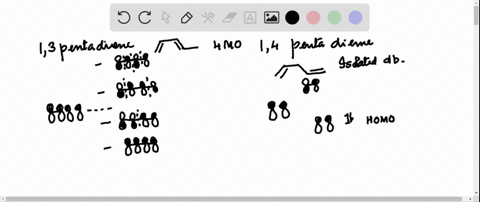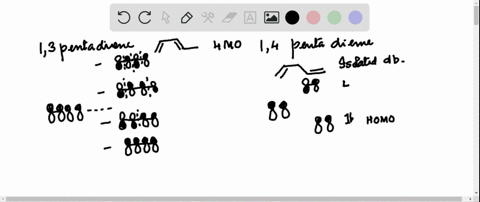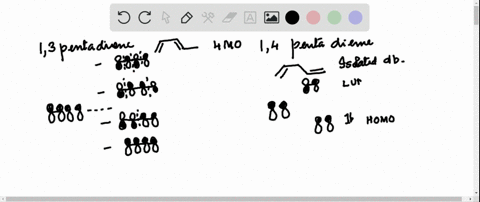
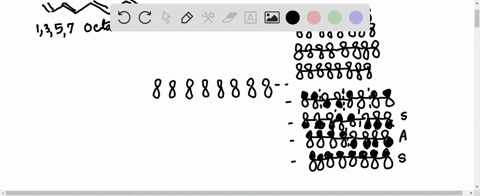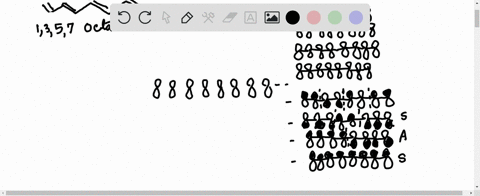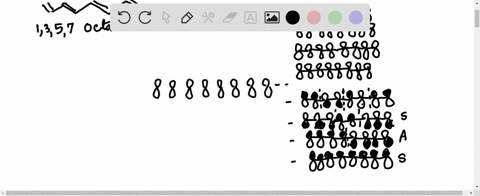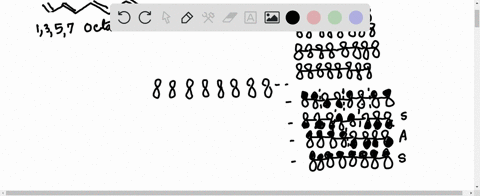
38 1 3 5 7-octatetraene molecular orbital diagram
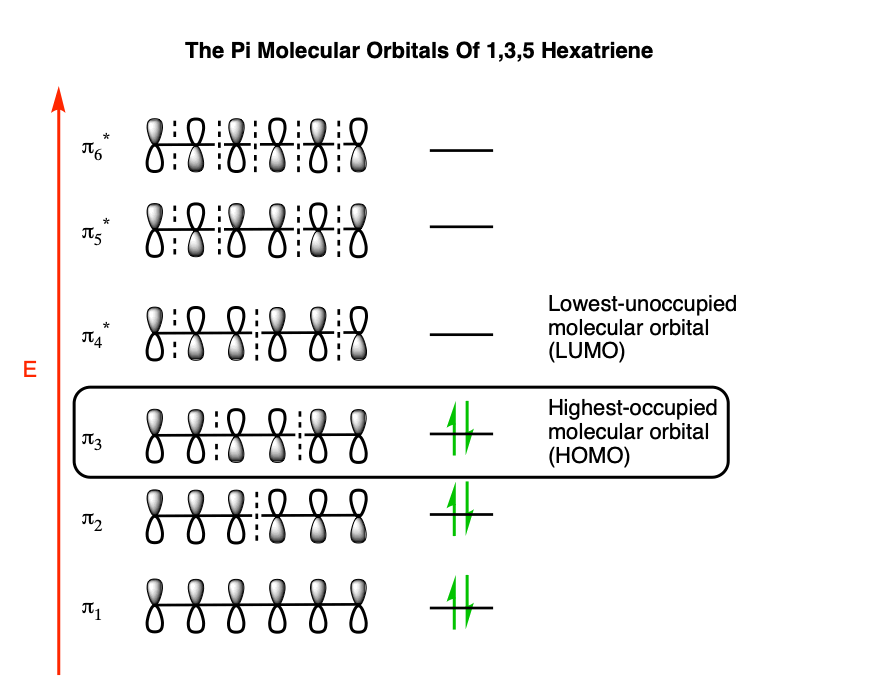
Pi Molecular Orbitals of 1,3,5-Hexatriene. With a single sigma bond separating the pi bonds of 1,3,5-hexatriene it is a conjugated system and some of the pi electron density will be delocalized between each of the C-C bonds, not just those written as double bonds in the Lewis structure. There are six adjacent carbon atoms involved in the pi ...
How many π molecular orbitals does 1,3,5,7-octatetraene have? Free. Unlocked . Multiple Choice . Unlock to view answer. Q16 Q16 Q16 . How many nodes does the highest energy molecular orbital of 1,3,5,7-octatetraene have? Free. Unlocked . Multiple Choice . Unlock to view answer. Q17 Q17 Q17 . In the allyl radical,which π molecular orbital is ...
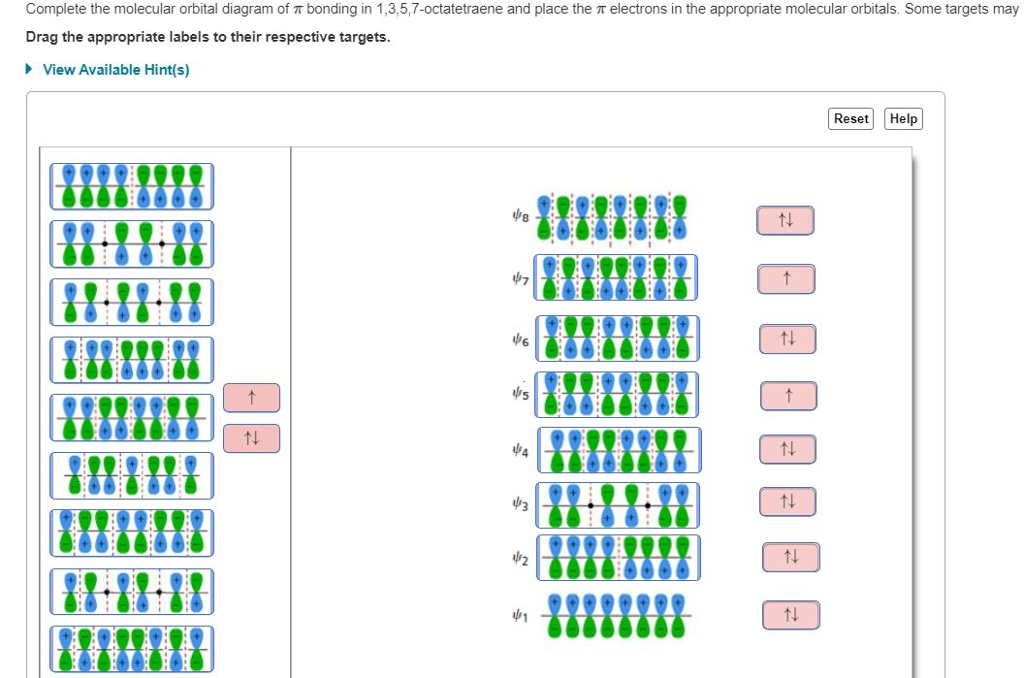
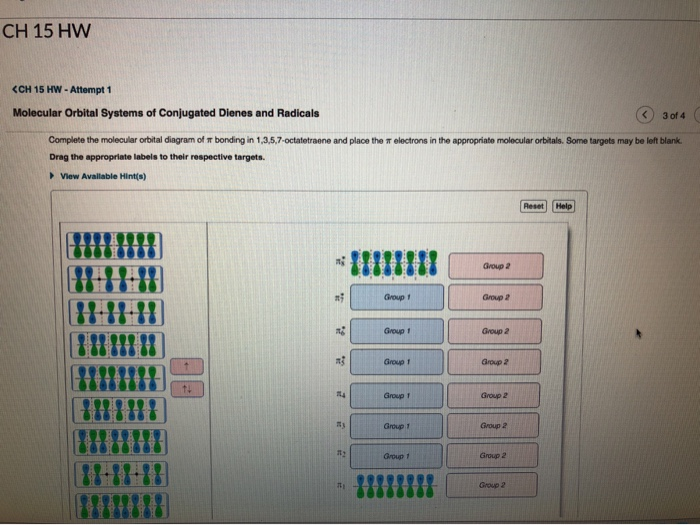
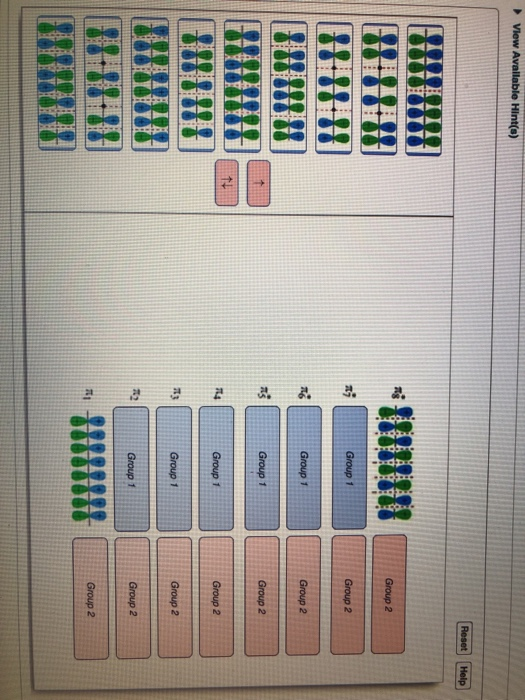
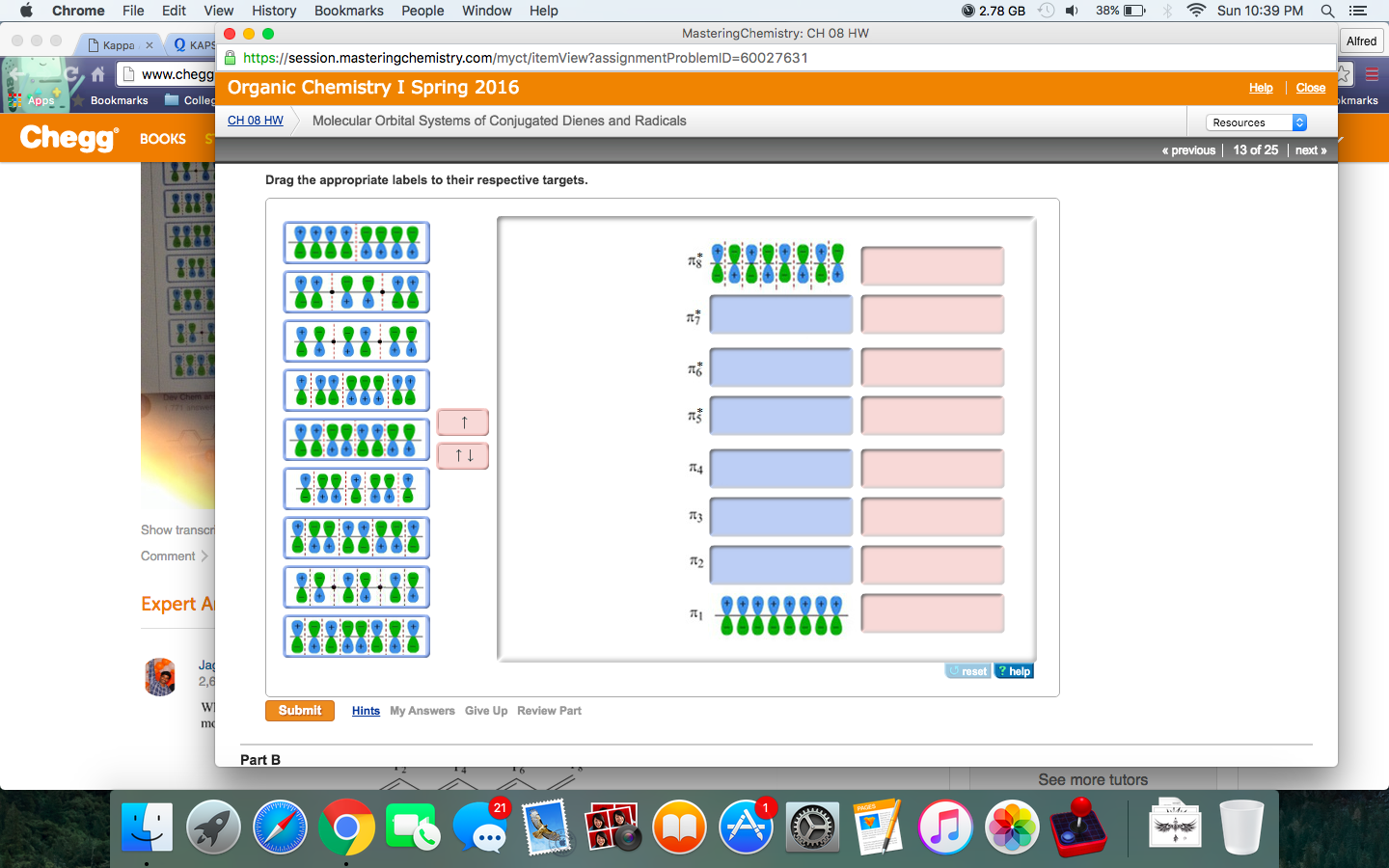
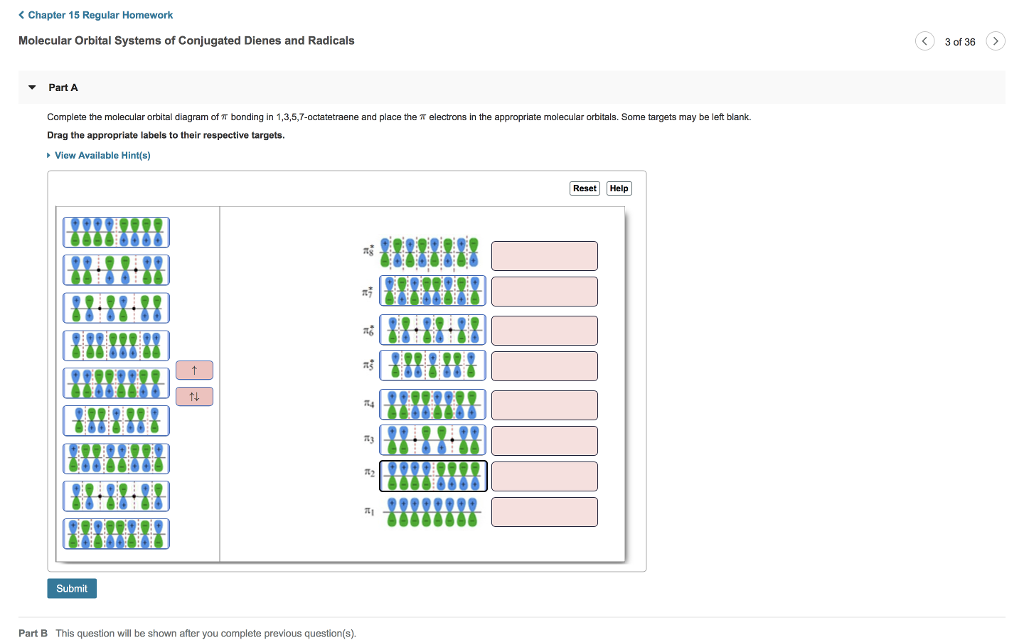
Complete the molecular orbital diagram of ? bonding in 1,3,5,7-octatetraene and place the ? electrons in the appropriate molecular orbitals. Some targets may be left blank. Drag the appropriate labels to their respective targets. help please
1 3 5 7-octatetraene molecular orbital diagram
π-Molecular Orbitals of 1,3,5,7-Octatetraene. How to Manipulate JSmol Structures or Click on the JSmol logo.. There may be a slight delay in the loading of orbitals.
Answer and Explanation: 1. Become a Study.com member to unlock this answer! Create your account. The structure of 1,3,5,7-octatetraene is shown below. According to the above structure for 1,3,5,7 ...
Electron‐energy‐loss and electron‐transmission spectra of (all‐E )‐1,3,5,7‐octatetraene were recorded with a trochoidal electron spectrometer.The energy‐loss spectra reveal two triplet states at 1.73 and 3.25 eV (0,0‐transitions), the UV‐active 1 1 B u state at 4.40 eV, and a higher‐lying singlet state at 6.04 eV. The 2 1 A g state recently reported to be the lowest excited ...
1 3 5 7-octatetraene molecular orbital diagram.
Structure, properties, spectra, suppliers and links for: 1,3,5,7,9-Decapentaene.
By comparison, cyclooctatetraene has eight electrons, six of these fill the molecular bonding orbitals and two occupy the degenerate pair of non-bonding orbitals. • Cyclooctatetraene must therefore lose or gain 2 electrons in order to have a closed shell structure. We have seen this already: the 1,3,5,7-tetramethyl-cyclooctatetraene dianion ...
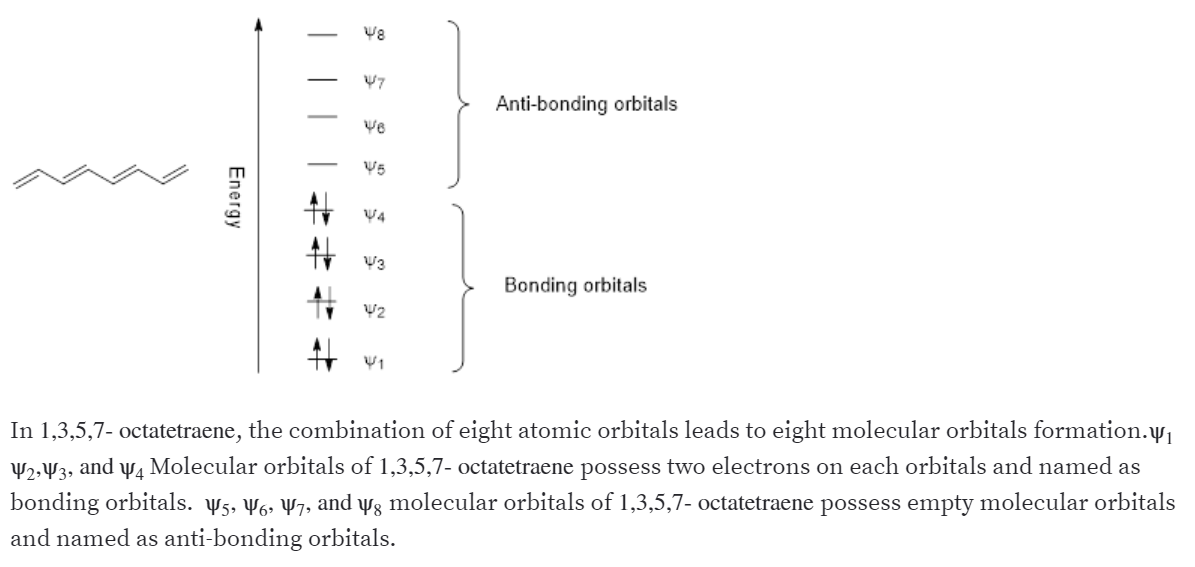
Molecular Orbital Energy Level Diagram of 1,3,5,7 Octatetraene:
Step 1. 1 of 4. a. 1,3,5,7- octatetraene has four. π \pi π. bonds. Therefore total number of. π \pi π. molecular orbitals are 8 - out of which 4 are bonding molecular orbitals and 4 are anti-bonding molecular orbitals. Step 2.
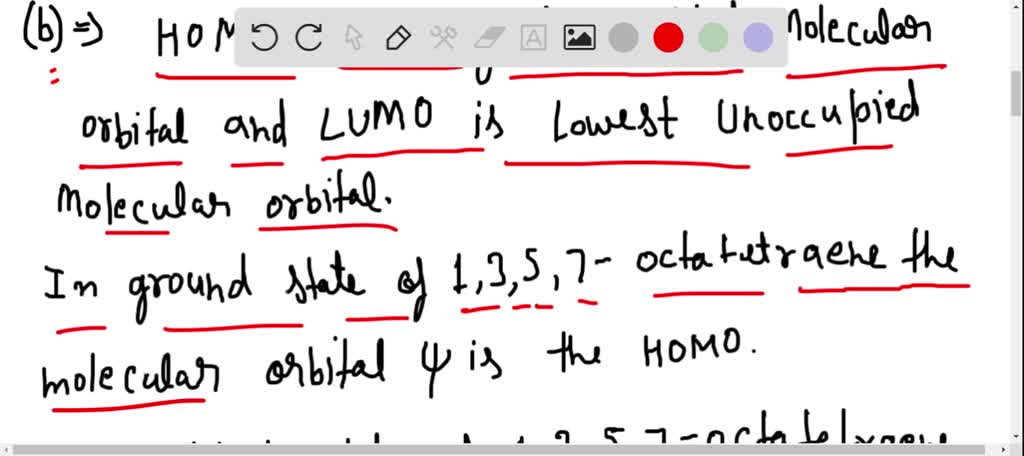
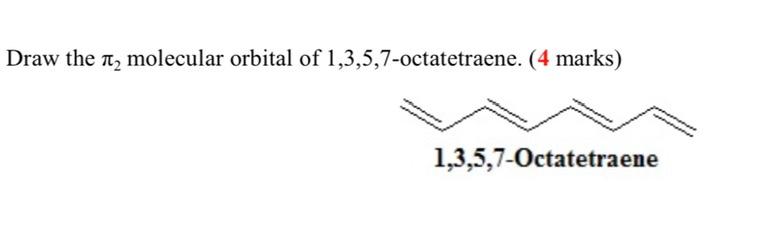
Chemistry. Chemistry questions and answers. Draw the MO diagram for 1,3,5,7-octatetraene showing the expected orbital symmetry for each MO. On your diagram label: Electron population of each MO HOMO, LUMO pi and pi* molecular orbitals (bonding and antibonding) presence of nodes with dashed lines.
each other (Figure 1.3). This destructive type of interaction is called a antibonding molecular orbital. An antibonding orbital is indicated by an asterisk 1*2. S* S H¬H H¬H 104 kcal>mol > The bonding molecular orbital and antibonding molecular orbital are shown in the molecular orbital diagram in Figure 1.4. In an MO diagram, the energies ...
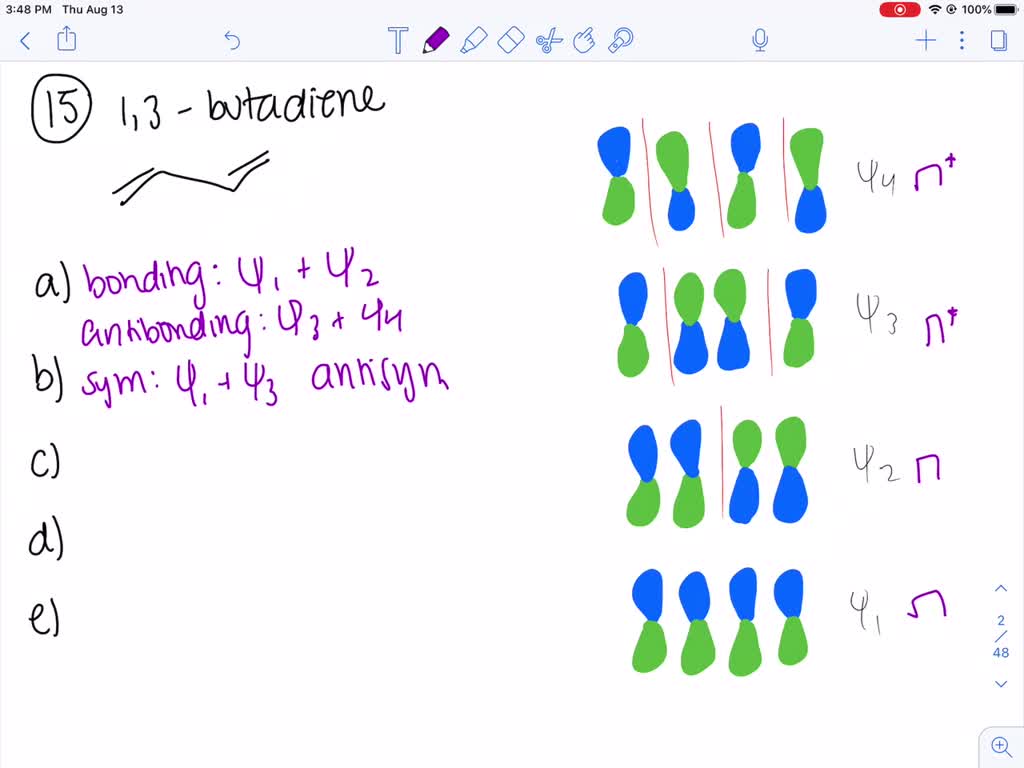
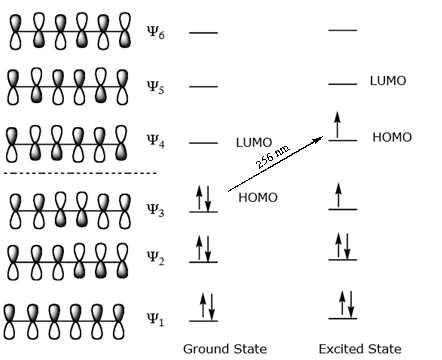
3. (10 points) For 1,3,5-hexatriene : a) Write an energy level diagram, indicating the number of pi molecular orbitals expected, their relative energies, the number of electrons expected in each orbital, and identifying the HOMO and LUMO. b) Draw approximate molecular orbital pictures of the HOMO and LUMO of 1,3,5-hexatriene, indicating orbital ...
When two team atomic orbital's come together such as these two, they conform a lower and energy bonding molecular orbital, which shows electron density between the atoms and then a pie to be star anti bio molecular orbital that shows no electron density between two atoms, and this would be higher in energy, the anti bonding.
1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C 8 H 8.It is also known as [8]annulene.This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of its stoichiometric relationship to benzene, COT has been the subject of much research and some controversy. ...
Predicted - ChemAxon. Predicted data is generated using the ACD/Labs Percepta Platform - PhysChem Module. Density: 0.8±0.1 g/cm 3. Boiling Point: 148.1±7.0 °C at 760 mmHg. Vapour Pressure: 5.4±0.1 mmHg at 25°C. Enthalpy of Vaporization:
1 2 5 3 6 4 1) Each of the MOs is a linear combination of 6 pz orbitals ⎛ cµ ⎞ ⎜ 1 µ ⎟ ⎜ c2 ⎟ 6 ⎜ cµ ⎟ ψµ = ∑cµ p z i → cµ = ⎜ 3 i µ ⎟ i=1 ⎜ c4 ⎟ ⎜ cµ ⎟ ⎜⎜ 5 ⎟ µ ⎟ ⎝ c6 ⎠ 2) It is relatively easy to work out the Hamiltonian. It is a 6by6 matrix. The first rule implies that every diagonal ...
Molecular Orbitals of BeH 2 • BeH 2 (D ∞h) 1σg22σ g 23σ u 2 Group orbitals of 2H's: ψs + ψs ψs - ψs H Be H z y Be 2 H 2px 2py 2pz 2s 1s s + s s - s-128 eV-8.2 eV 2.2 eV -13.5 eV D∞h ∞σv i Σg + 1 1 x2+y 2, z 2 Σu + 1 -1 z Πu 0 -2 (x,y) σσσσg + σσσσu + σσσσg + σσσσu + ππππu 1σσσσg ππππu 2σσσσg ...

A how many pi molecular orbitals does 1357 octatetraene have b what is the designation of its math 2
Octa-1,3,5,7-tetraene | C8H10 | CID 137023 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities ...
1. Even Number of Carbon Atoms The next higher conjugated system having an even number of carbon atoms in it would be a conjugated triene, such as 1,3,5-hexatriene. You should anticipate by now that with six carbon atoms there would be six pi MO's, three of which would be bonding and three antibonding.
Para-nitroaniline and 1,3,5,7-all-trans-octatetraene are chosen as test compounds. It turns out that it is necessary to include at least single and double excitations (CISD) and to have active windows containing the complete scheme of π- and n-type orbitals to get converged CI solutions.
Natural orbitals for chemical valence (NOCV) were used to describe bonding in conjugated π-electron molecules. The 'single' C-C bond in trans-1,3-butadiene, 1,3-butadiene-1,1,4,4-tetra-carboxilic acid, 1,3,5,7-octatetraene, and 11-cis-retinal was characterized.In the NOCV framework, the formation of the σ-bond appears as the sum of two complementary charge transfer processes from each ...
1,3,5,7-octatetraene molecular orbitals (octa-1,3,5,7-tetraene) The pi-molecular orbitals of open chain CH 2 =CH-CH=CH-CH=CH-CH=CH 2. Posted by Unknown at 7:52 AM.
Answer the following questions for the pi molecular orbitals of 1357 octatetraene a how many pi mo 2
Complete the molecular orbital diagram of ? bonding in 1,3,5,7-octatetraene and place the ? electrons in the appropriate molecular orbitals. Some targets may be left blank. Drag the appropriate labels to their respective targets. Jul 22 2021 03:30 AM. Solution.pdf.
We have applied the new method to compute excitation energies in conjugated systems of π-electrons such as trans-1,3-butadiene, trans,trans-1,3,5-hexatriene, and all-trans-1,3,5,7-octatetraene. The ordering of the excited states is correctly reproduced by the DFT-SSMRPT calculations.
Answer the following questions for the $\pi$ molecular orbitals of 1,3,5,7 -octatetraene: a. How many $\pi$ MOs does the compound have? b. Which are the bonding MOs and which are the antibonding MOs? c. Which MOs are symmetric and which are asymmetric? d. Which MO is the HOMO and which is the LUMO in the ground state? e.
Click here👆to get an answer to your question ️ 1,3,5,7 - Octatetraene contains X σ - bonds and Y pi - bonds. X and Y are: Join / Login >> Class 11 >> Chemistry >> Chemical Bonding and Molecular Structure >> Valence Bond Theory >> 1,3,5,7 ... It is formed by the head-on overlap of two atomic orbitals.
1,3,5,7-Octatetraene | C8H10 | CID 12576133 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities ...
If [1,3] or [1,7] hydrogen shifts were taking place, the deuterium atoms would be distributed equally among all eight carbon atoms. On prolonged heating, or at higher temperatures these cyclooctatrienes undergo electrocyclic ring opening to 1,3,5,7-octatetraene and reclosure to vinyl-1,3-cyclohexadienes.

A how many mos does 1357 octatetraene have b what is the designation of its mathrmhomoleftpsi_1 psi_




![Solved 13 8. [8 marks] (a) The structure of | Chegg.com](https://media.cheggcdn.com/media%2F9d4%2F9d482eab-0224-4901-aab1-5142946fd6a8%2FphpYc683V.png)









0 Response to "38 1 3 5 7-octatetraene molecular orbital diagram"
Post a Comment