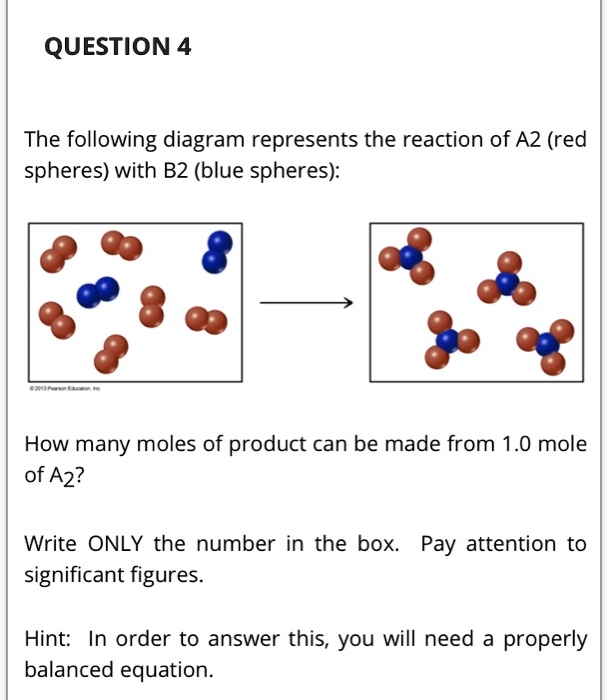
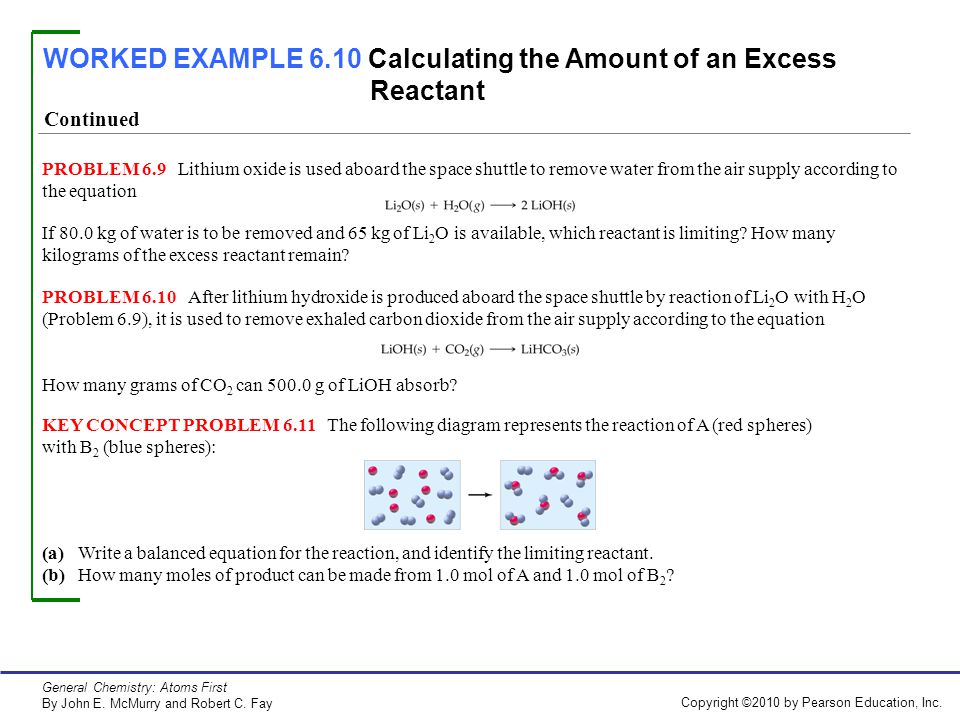
38 the following diagram represents the reaction of a (red spheres) with b2 (blue spheres).
7.29 Consider a reaction represented by the following spheres: Which sphere represents a metal and which a nonmetal? Explain. Metals form cations, and since cations are smaller than their parent atom, the first (red) sphere will be the metal atom. Nonmetals form anions, and since anions are larger than their parent atom, the second (blue ... Find an answer to your question Let a represent the red spheres and b represent the blue spheres. write a balanced equation for the equilibrium reaction. Maddie1201 Maddie1201 03/31/2018 ... I have attached the diagram related to this question. Answer: 5A₂ + 5B .....> 4A₂B + A₂ + B
SOLVED:The reaction of A (red spheres) with B (blue spheres) is shown in the following diagram: Problem. Cytosine, a constituent of deoxyribonucleic acid …. 03:51. View Full Video.

The following diagram represents the reaction of a (red spheres) with b2 (blue spheres).
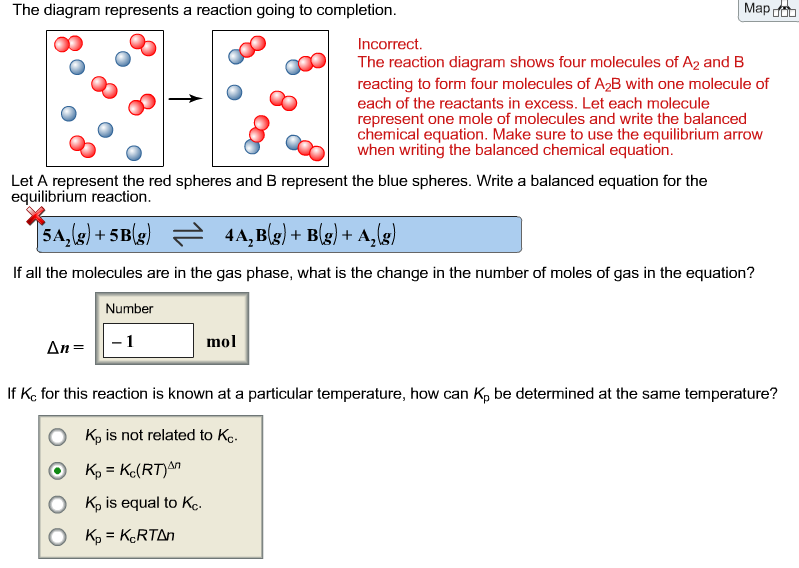
2.2 The following diagram is a representation of 20 atoms of a fictitious element, which we will call nevadium (Nv). The red spheres are 293Nv, and the blue spheres are 295Nv. (a) Assuming that this sample is a statistically representative sample of the element, calculate the percent abundance of each element. The following diagram represents the reaction of a red spheres with b2 blue spheres. B write a balanced equation for the reaction. How many moles of product can be made from 10 mol of a 2 and 10 mol of b 2. All the reactions proceed according to the general balanced chemical reaction abab red spheres represent a atoms blue spheres are b atoms ... Problem 4. The following diagram represents a reaction shown going to completion. (a) Letting A = red spheres and B = blue spheres, write a balanced equation for the reaction. (b) Write the equilibrium-constant expression for the reaction. (c) Assuming that all of the molecules are in the gas phase, calculate Δ n, the change in the number of ...
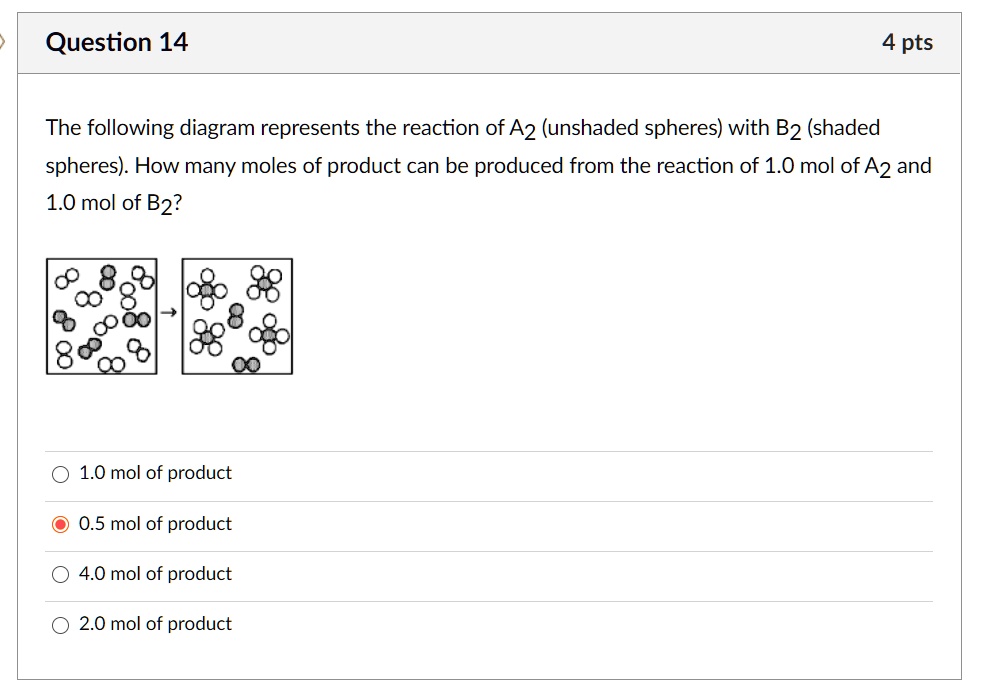
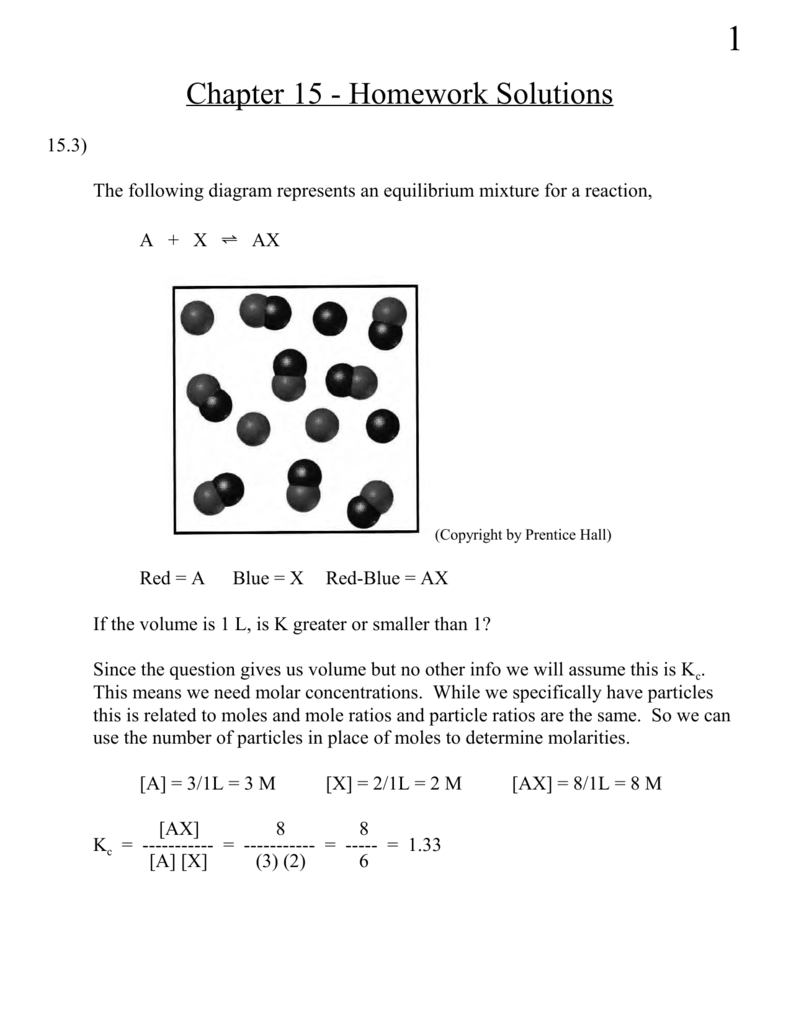
The following diagram represents the reaction of a (red spheres) with b2 (blue spheres).. Problem 15.2 - Enhanced - with Feedback The following diagrams represent a hypothetical reaction A → B with A represented by red spheres and B represented by blue spheres. The sequence from left to right represents the system as time passes. 1 2 3 .5 In which diagram(s) is the system in equilibrium? Check all that apply. 15.3 The following diagram represents an equilibrium mixture produced for a reaction of the type A + X AX. If the volume is 1 L, is K greater or smaller than 1? [Section 15.2] 15.4 The following diagram represents a reaction shown going to completion. (a) Letting A = red spheres and B = blue spheres, write a balanced equation for the reaction. Problem Details. The reaction between reactant A (blue spheres) and reactant B (red spheres) is shown in the following diagram: Based on this diagram, which equation best describes the reaction? (a) A 2 + B → A 2 B. (b) A 2 + 4B → 2AB 2. (c) 2A + B 4 → 2AB 2. (d) A + B 2 → AB 2. 2. The following diagram represents the reaction of A2 molecule (shaded spheres) with B2 Molecule (unshaded spheres) to form AB3 molecules. Identify the limiting reactant and write a balanced chemical equation for the reaction. Start End A B a) A2 is the limiting reactant: A + 3 B AB3 b) A2 is the limiting reactant: A2 + 3 B2 2 AB3
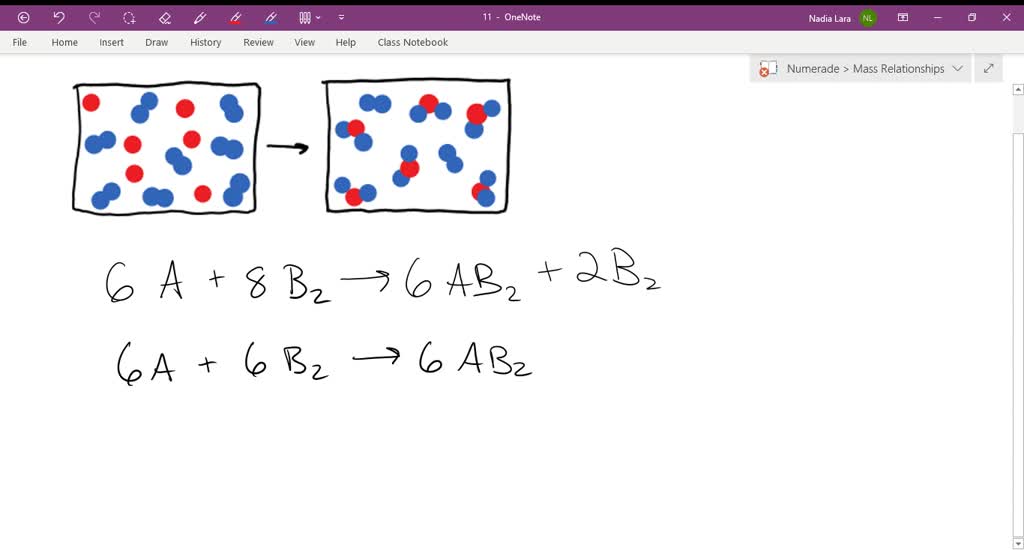
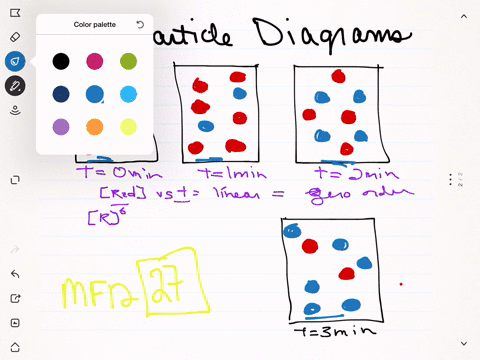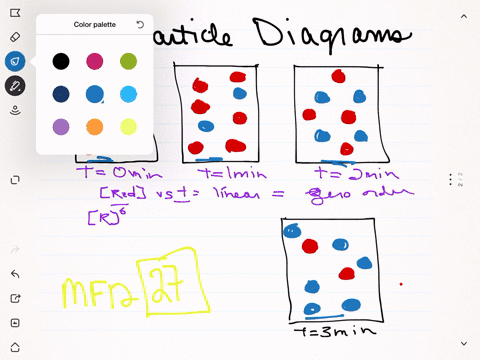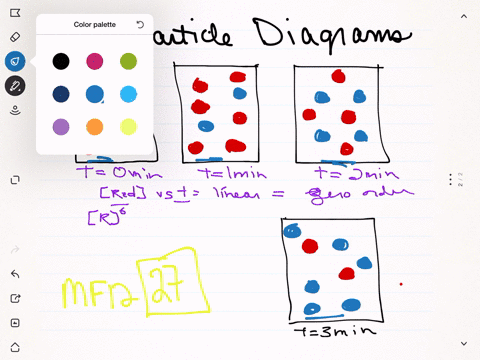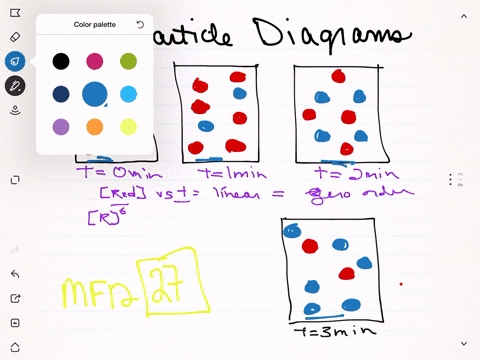
The following diagram represents the collection of elements formed by the decomposition of a compound. The blue spheres represent nitrogen atoms and the red spheres represent oxygen atoms, what was the empirical formula of the original compound? 2 1. N6O12 2. N3O6 3. NO2 2 The following diagrams represent the reaction of (red spheres) with (blue spheres) to give specific products. Write a balanced equation for each reaction based on the information in the diagram. Problem: The following diagram represents a reaction shown going to completion. Each molecule in the diagram represents 0.1 mol and the volume of the box is 1.0 L. Letting A = red spheres and B = blue spheres, write a balanced equation for the reaction. Express your answer as a chemical equation. 2. The diagram below shows steps in the exothermic chemical reaction of bromomethane with hydroxide to form methanol and bromide ion. The black spheres are carbon atoms, the white spheres are hydrogen atoms, the red spheres are oxygen atoms, and the blue dots are bromine atoms. A B Write a balanced chemical equation to represent the reaction.
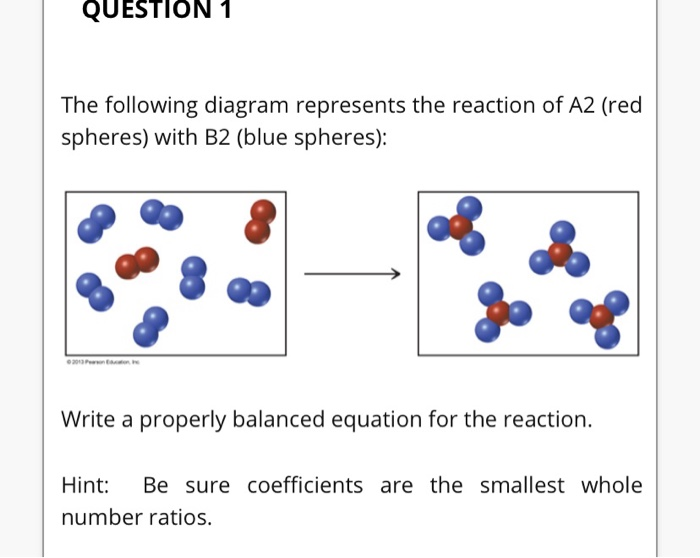
The following diagram represents the reaction of A2 (red spheres) with B2 (blue spheres): Write a balanced equation for the reaction. Identify the limiting reactant. The following diagram represents a chemical reaction in which the red spheres are oxygen atoms and the blue spheres are nitrogen atoms. (a) Write the chemical formulas for the reactants and products. (b) Write a balanced equation for the reaction. (c) Is the diagram consistent with the law of conservation of mass? Answered: The following diagram represents the… | bartleby. Hit Return to see all results. Science. Chemistry Q&A Library The following diagram represents the reaction of A (red spheres) with B2 (blue spheres). Write a balanced equation for the reaction. 3.3 The following diagram represents the collection of elements formed by a decomposition reaction. (a ) If the blue spheres rep-resent N atoms and the red ones represent O atoms, what was the empirical formula of the original compound? (b ) Could you 3.5 Glycine, an amino acid used by organisms to make proteins,
Created Date: 1/29/2013 11:59:35 AM
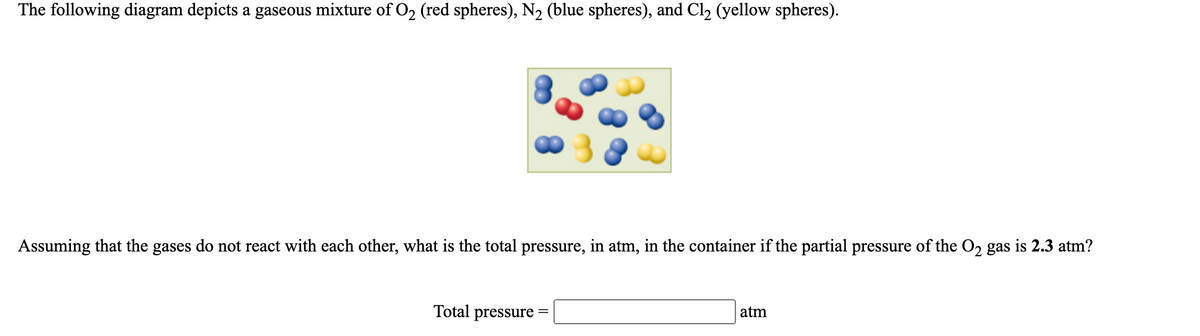
Balance each of the following by adding coefficients: If red spheres represent oxygen atoms and blue spheres represent nitrogen atoms a. Write the formula for each of the reactants and products. b. Write a balanced equation for the reaction. If blue spheres represent nitrogen atoms and purple spheres represent iodine, a.
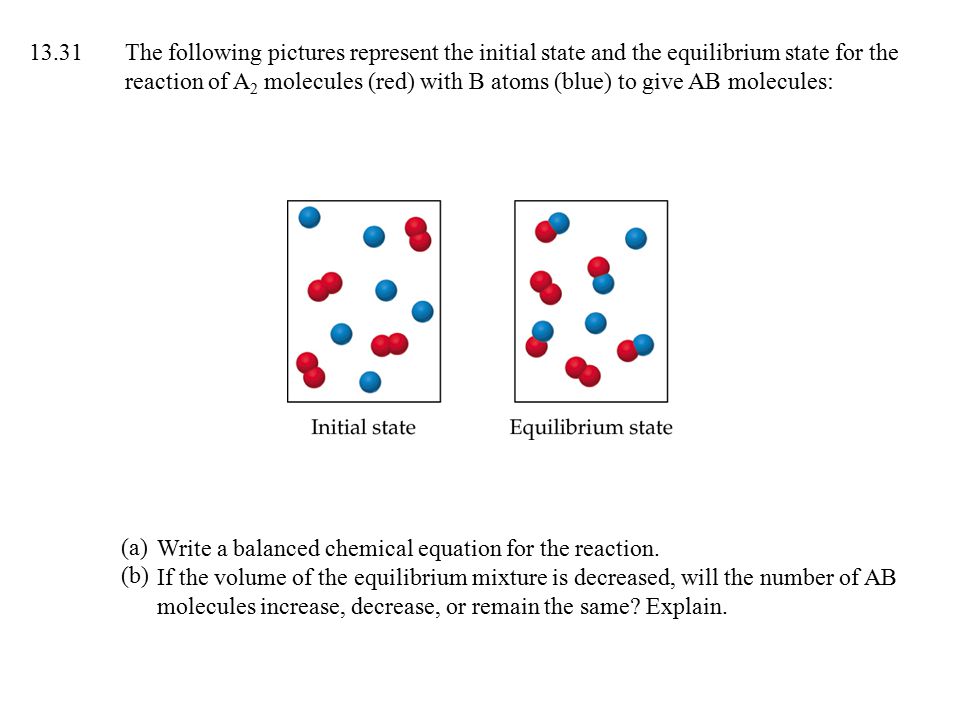
The following diagram represents a reaction shown going to completion. (a) Letting A = red spheres and B = blue spheres, write a balanced equation for the reaction.(b)Write the equilibrium- constant expression for the reaction.(c) Assuming that all of the molecules are in the gas phase, calculate ∆n, the change in the number of gas molecules that accompanies the reaction.
15.4 The following diagram represents a reaction shown going to completion. Each molecule in the diagram represents 0.1 mol and the volume of the box is 1.0 L. (a) Letting A = red spheres and B = blue spheres, write a balanced equation for the reac-tion. (b) Write the equilibrium-constant expression for the re-
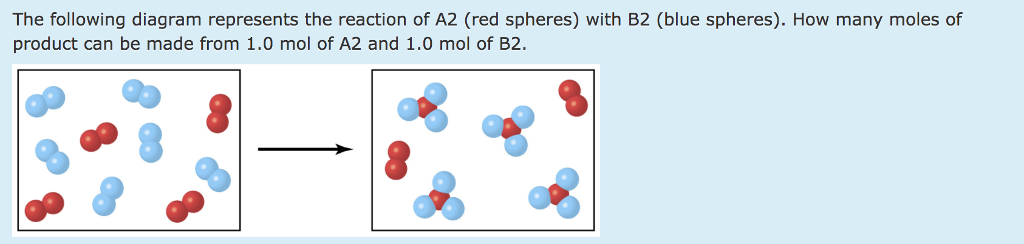
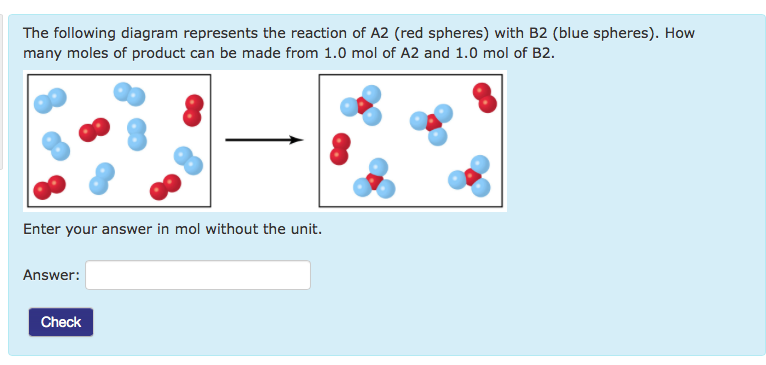
The following diagram represents the reaction of A (red spheres) with B2 (blue spheres): (a) Write a balanced equation for the reaction, and identify the limiting reactant. (b) How many moles of product can be made from 1.0 mol of A and 1.0 mol of B2? Step-1 (a) Balanced equation is
3.3 The following diagram represents the collection of elements formed by a decomposition reaction. (a) If the blue spheres represent N atoms and the red ones represent O atoms, what was the empirical formula of the original compound? (b) Could you draw a diagram representing the molecules of the compound that had been decomposed? Why or why not?
The following diagram represents a reaction shown going to completion. Each molecule in the diagram represents 0.1 mol and the volume of the box is 1.0 L. Part A Letting A = red spheres and B = blue spheres, write a balanced equation for the...
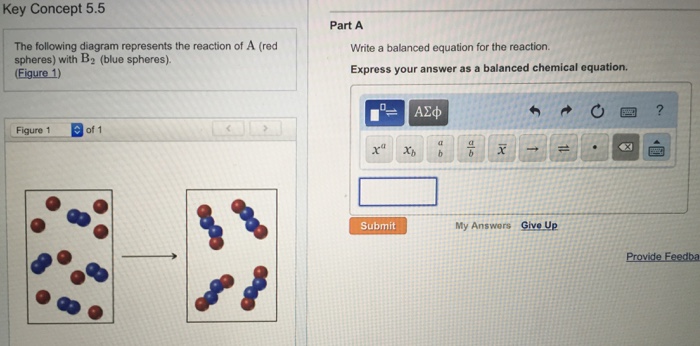
The following diagram represents the reaction of A (red spheres) with B2 (blue spheres). (Figure 1) Part A Write a balanced equation for the reaction. Express your answer as a balanced chemical equation. ΑΣΦ ? Figure 1 of 1 Submit Previous Answers Request Answer Provide Feedback
The diagram represents the collection of elements formed by a decomposition reaction. (a) If the blue spheres represent N atoms and the red ones represent O atoms, what was the empirical formula of the original compound? (b) Could you draw a diagram representing the molecules of the compound that had been decomposed? Why or why not?
43. (4 pts) The following diagram represents the reaction of A 2 (gray spheres) with B 2 (white spheres): a. Write a balanced chemical equation for the reaction; A2 + 3 B 2 2 AB 3 b. Identify the limiting reactant; Limiting reactant: B 2 Reactant in excess: A 2 c. How many moles of product can be made from 1.0 mol of A 2 and 1.0 mol of B 2?
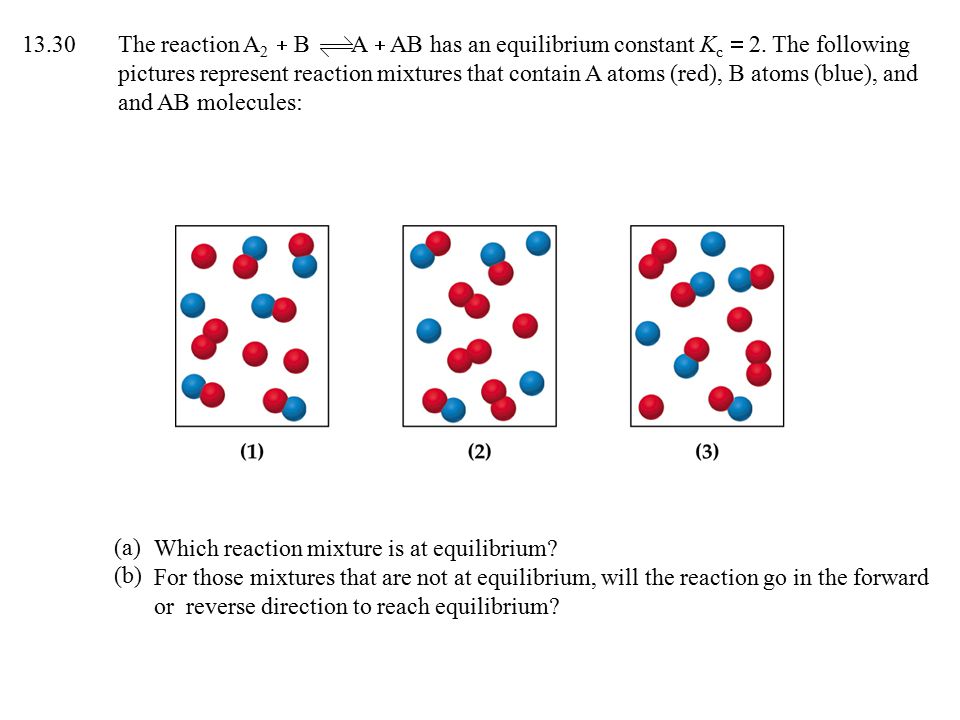
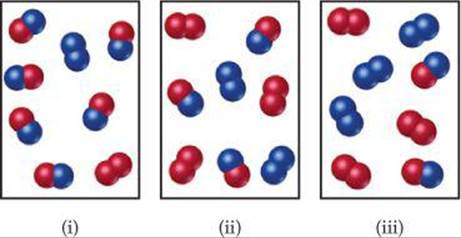
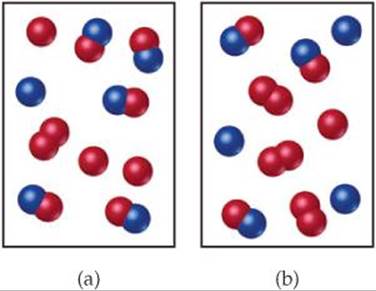
The reaction A2+B2−⇀↽−2AB has an equilibrium constant Kc=1.5. The diagrams represent reaction mixtures where A is represented by red spheres and B is represented by blue spheres. For each diagram, determine whether the reaction mixture is at equilibrium, or the direction in which the reaction will proceed to reach equilibrium.
Problem 4. The following diagram represents a reaction shown going to completion. (a) Letting A = red spheres and B = blue spheres, write a balanced equation for the reaction. (b) Write the equilibrium-constant expression for the reaction. (c) Assuming that all of the molecules are in the gas phase, calculate Δ n, the change in the number of ...
The following diagram represents the reaction of a red spheres with b2 blue spheres. B write a balanced equation for the reaction. How many moles of product can be made from 10 mol of a 2 and 10 mol of b 2. All the reactions proceed according to the general balanced chemical reaction abab red spheres represent a atoms blue spheres are b atoms ...
2.2 The following diagram is a representation of 20 atoms of a fictitious element, which we will call nevadium (Nv). The red spheres are 293Nv, and the blue spheres are 295Nv. (a) Assuming that this sample is a statistically representative sample of the element, calculate the percent abundance of each element.



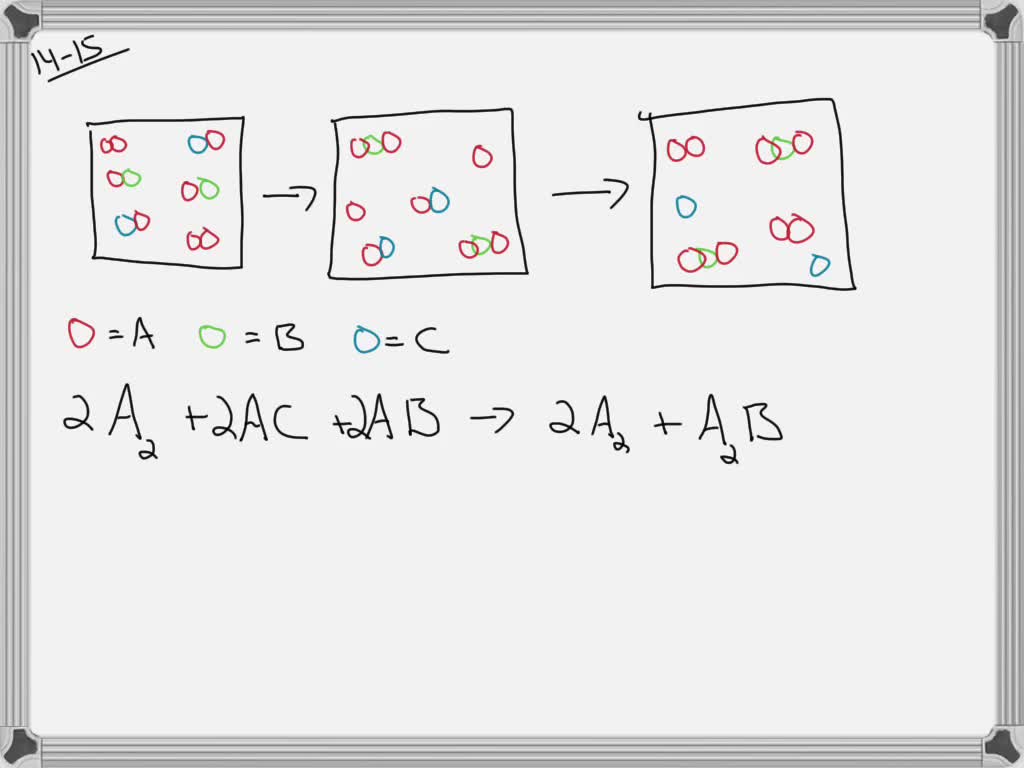




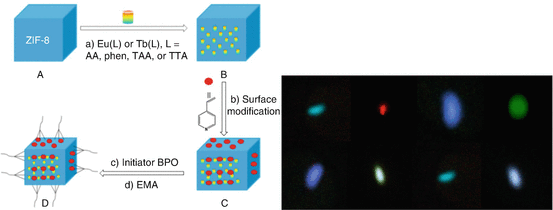




0 Response to "38 the following diagram represents the reaction of a (red spheres) with b2 (blue spheres)."
Post a Comment