39 Orbital Diagram Of Silicon
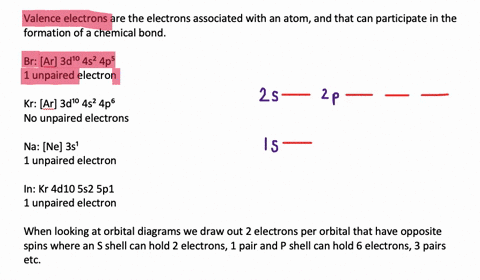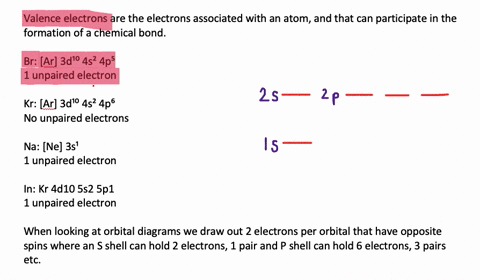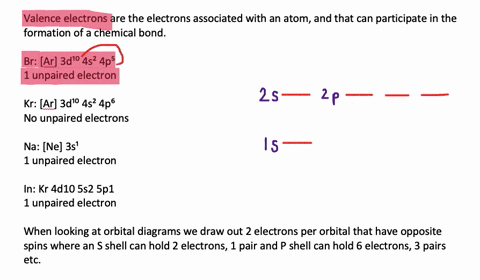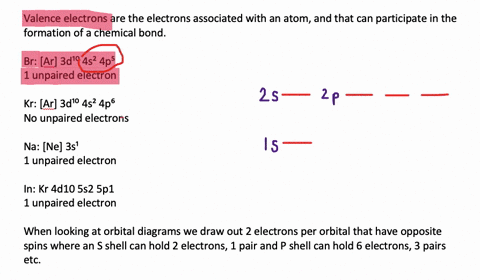
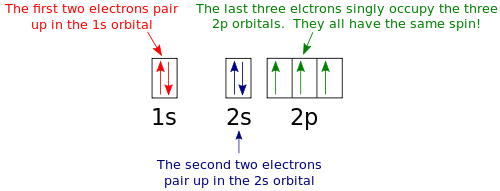
Silicon Electron Configuration (Si) with Orbital Diagram How Many Valence Electrons are in Silicon. There are 4 electrons in the outer shell of Silicon so the number of valence electrons in silicon is 4. Silicon Orbital Diagram. Orbit diagram consists of a pair of electrons of the atom in the box i.e. Orbit diagram helps to define the ground-state electron configuration is an easy form. Electron Configuration for Silicon (Si) - UMD In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two ...
Orbital Diagram For Vanadium (V) | Vanadium Electron ... 2021-02-18 · Just like there are 5 valence electrons for the element Vanadium. Similarly, every element will have its own valence electrons and many more. You can refer our article to those users or your friends who are looking for the information related to the Vanadium Electron Configuration of valence electrons as the good thing about our article is that it is available free …
Orbital diagram of silicon
Carbon Bohr Model - How to draw Bohr diagram for Carbon(C ... S orbital can hold maximum of 2 electrons and P orbital can hold maximum of 6 electrons. FAQ. How many electron shells a Carbon Bohr model contains? Electron shell also called energy level, you can find the number of electron shells for an element by knowing its period number in the periodic table. The elements or atoms in the first period of the periodic table have one energy … Electron configuration of silicon (Si), orbital diagram ... Orbital Diagram, electron configuration, and the noble gas notation for a silicon (Si) atom. iperiodictable.com › nitrogen-electron-configurationOrbital Diagram For Nitrogen (N) | Nitrogen Electron ... Ground State Electron Configuration For Nitrogen. When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s 2 2s 2 2p 3.Below you can get the full image representation which will help you to understand the topic more easily.
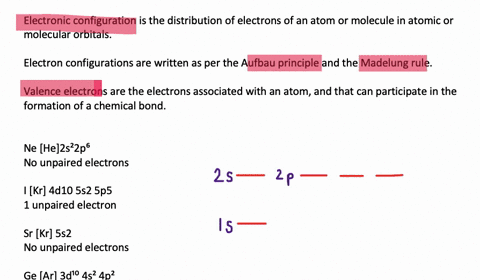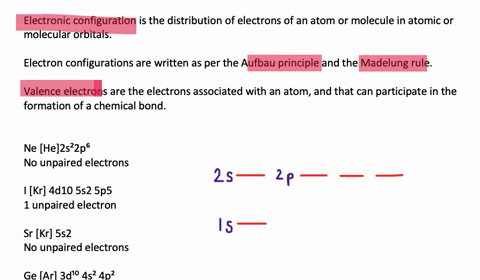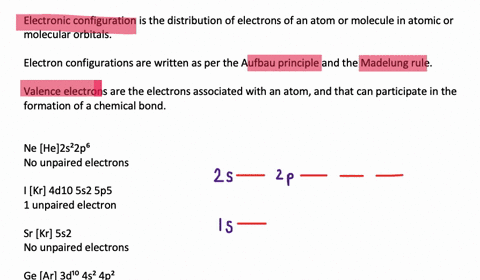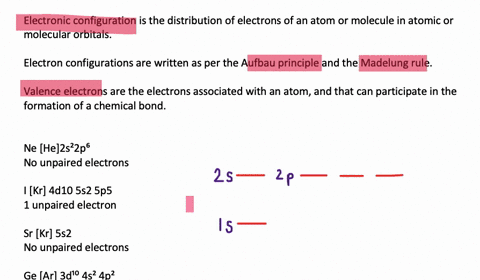
Orbital diagram of silicon. Silicon Orbital Diagram - ViralListClub.com Silicon orbital diagram. Show the orbital-filling diagram for S sulfur. It is a hard brittle crystalline solid with a blue-grey metallic lustre and is a tetravalent metalloid and semiconductorIt is a member of group 14 in the periodic table. Also the crystalline form is used in semiconductors. Three rules are useful in forming orbital diagrams. Neon(Ne) electron configuration and orbital diagram Neon(Ne) is the 10th element in the periodic table and its symbol is 'Ne'. This article gives an idea about the electron configuration of neon and orbital diagram, period and groups, valency and valence electrons of neon, application of different principles. › orbitaldOrbital Diagrams Chemistry Tutorial - AUS-e-TUTE An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s. Bonding in Metals and Semiconductors - lardbucket Figure 12.21 The Molecular Orbital Energy-Level Diagram for a Linear Arrangement of n Atoms, Each of Which Contains a Singly Occupied s Orbital. This is the same diagram as Figure 9.35 "Bonding in Ozone", with the addition of the far right-hand portion, corresponding to n = 30 and n = ∞. As n becomes very large, the energy separation between adjacent levels becomes so small that a single ...
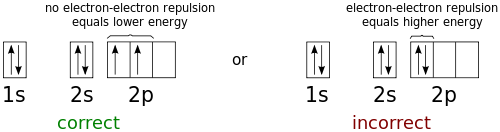
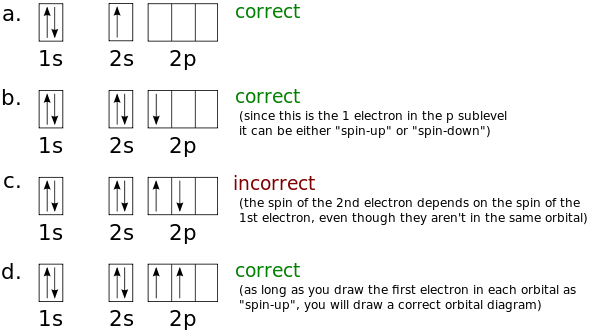
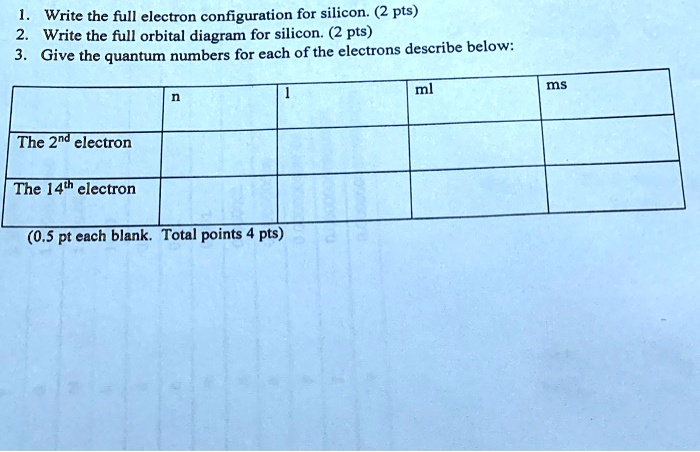
› ~seshadri › 2018_218Band Structures and the Meaning of the Wave Vector k with its neighbors. Strong orbital interactions lead to a more disperse band (green curve) which becomes successively flatter with weaker interactions (blue and red curves). when m= nwhere is simply the energy of an electron in one atomic orbital and Z ˜ m H˜ n= (21) when m= (n 1) where is the interaction energy. How many unpaired electrons are found in the ground state ... Orbital diagrams are pictorial descriptions of the electrons in an atom. Three guidelines are helpful in forming orbital diagrams. In accordance with the Auf Bau Precept, every electron occupies the bottom power orbital. You leap up somewhat bit in power and we get the 2s orbital that make it the 2p sublevel. Mercury (planet) - Wikipedia Mercury is the smallest planet in the Solar System and the closest to the Sun.Its orbit around the Sun takes 87.97 Earth days, the shortest of all the Sun's planets. It is named after the Roman god Mercurius (), god of commerce, messenger of the gods, and mediator between gods and mortals, corresponding to the Greek god Hermes (Ἑρμῆς). Like Venus, Mercury orbits the Sun within … 6.4 Electronic Structure of Atoms (Electron Configurations ... The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).
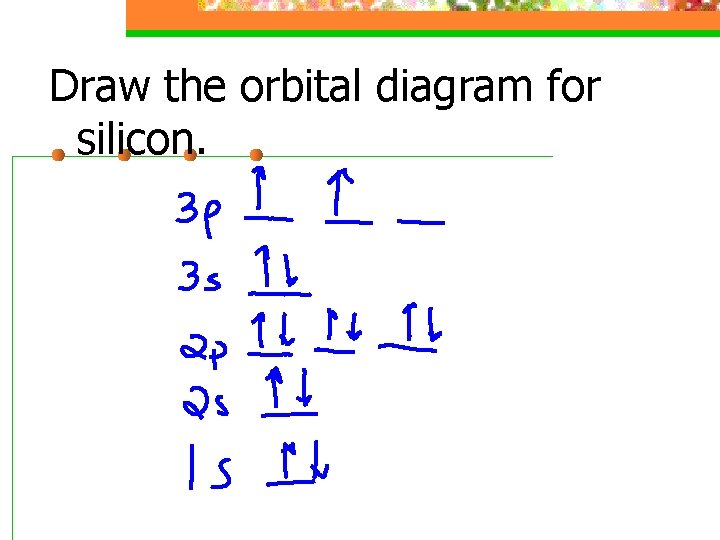
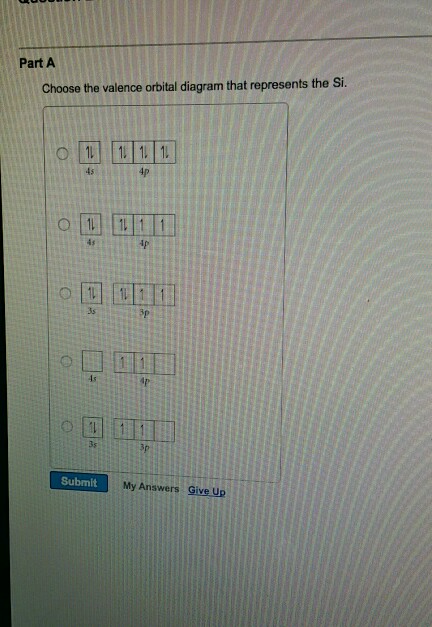
Aluminum-Silicon Alloys :: Total Materia Article Aluminum-silicon alloys without copper have good corrosion resistance in most reagents; only in alkaline solutions which attack silicon as well as aluminum their performance is poor. Copper reduces appreciably the corrosion resistance and so does iron, unless corrected with manganese or chromium. Zinc up to 2-3% has no effect. The electron configuration of silicon needs to be written ... Given: Silicon atom. An orbital diagram refers as the arrangement of the electrons in an atom. In the orbital diagram, the each orbital is shown as square and in a sublevel they are represented next to each other in a horizontal way. Silicon [N e] 3 s ² 3 p ² What is the orbital notation for silicon? - Answers What is the orbital notation for silicon? Its hard to put this on a screen with a regular key board. But the 1's at the top represent arrows in the pared up arrows imagine the first one pointing ... sciencing.com › calculate-valency-2790How to Calculate Valency - Sciencing Feb 10, 2020 · Valency is a measure of the ability of an atom to bond with other atoms. The higher the number of valent electrons, the more reactive the atom or molecule is. Electrons will occupy the most stable position first. The inner orbital holds up to 2 electrons. The next orbital holds up to 8 electrons.
Silicon(Si) electron configuration and orbital diagram Silicon(Si) is the 14th element in the periodic table and its symbol is 'Si'. Silicon is a semiconductor material. The electron configuration of silicon and the orbital diagram is the main topic of this article. Also, valency and valence electrons of silicon, various reactions, and compound formation, bond formation have been discussed ...
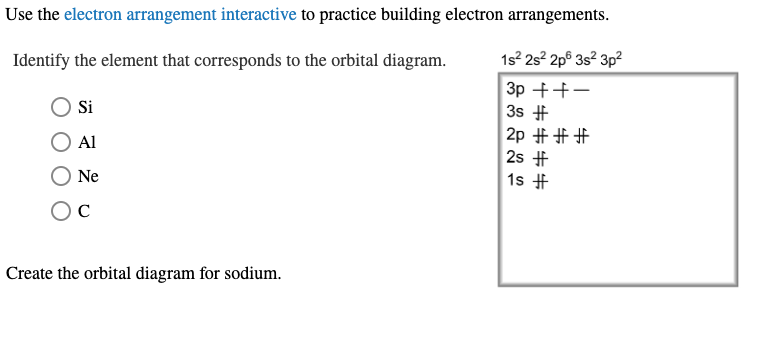
Solved examine the orbital diagram for the ground state ... Question: examine the orbital diagram for the ground state electron configuration of silicon choose best dxplanataion for why this orbital diagram is incorrect foe the ground state of silicon. This problem has been solved! See the answer See the answer See the answer done loading.
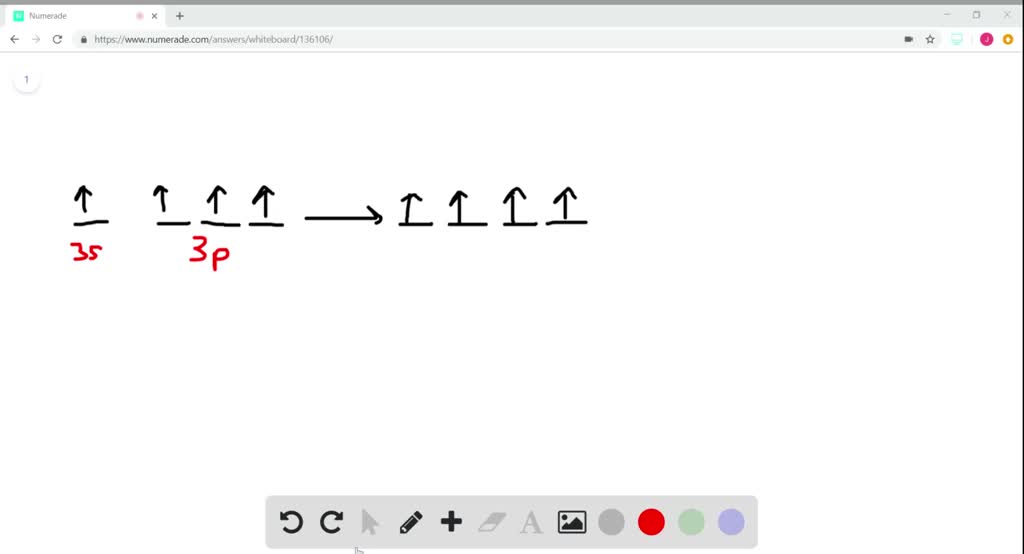
SiF4 Lewis Structure, Molecular Geometry, Hybridization ... But, the silicon atom forms four sigma bonds as per the Lewis structure of silicon tetrafluoride and VSEPR theory. Hence, one of the electrons from the 3s orbital excite to the 3p orbital of the silicon atom and provide four unpaired electrons for the formation of four sigma bonds with four fluorine atoms.
What is the orbital diagram for silicon? - Studyrankersonline In writing the electron configuration for Silicon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital.
PDF Home - Chemistry 9.7 The orbital diagram below presents the final step in the CQ formation of hybrid orbitals by a silicon atom. (a) Do you think one or more electrons have been promoted? Why or why not? (b) What type of hybrid orbitals are being produced in this hybridization? [Section 9.5] 9.8 Consider the hydrocarbon drawn below. (a) What is the
What is the orbital diagram for silicon? | Study.com The Orbital Diagram for Silicon: The orbital diagram for an element shows the electron distribution of the electrons, and the correct pairing of electrons with respect to electron spin.
1.4: Electron Configurations & Electronic Orbital Diagrams ... Example 1: Hydrogen and Helium. The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers and electron orbitals, we can conclude that these four quantum numbers refer to the 1s subshell.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15 ...
What is the orbital diagram for Silicon? - Answers An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represent the electrons in ...
Solar geoengineering - Wikipedia Solar geoengineering, or solar radiation modification (SRM) is a proposed type of climate engineering in which sunlight (solar radiation) would be reflected back to space to limit or reverse human-caused climate change.It is a possible quick emergency measure to limit overheating while greenhouse gases already in the atmosphere decay or are removed, not a substitute for …
Silicon Electron Configuration | Orbital Diagram For ... Silicon Orbital Diagram. Well, the orbital diagram of Silicon Electron Configuration is the best source of information if you are willing to study the chemical bonding of silicon. This is a kind of qualitative diagram that explains the whole combining process of the element in the best possible manner.
Orbital Diagram For Arsenic - schematron.org Orbital Diagram For Arsenic. Because the 4p section has 3 orbitals, but Arsenic ends with 4p3. It'll want to leave as few orbitals empty, so you have three arrows pointing up. The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org!
Silicon Electron Configuration - electron configuration of ... Here are a number of highest rated Silicon Electron Configuration pictures upon internet. We identified it from reliable source. Its submitted by direction in the best field. We acknowledge this kind of Silicon Electron Configuration graphic could possibly be the most trending subject similar to we ration it in google help or facebook.
› science › matterElectron Shells and Orbitals - TechnologyUK Possible values for the magnetic quantum number m for a p orbital are -1, 0 and +1 (since ℓ is equal to one), which means that there can be three p orbitals in any of the electron shells except 1n. Once the s orbital in each electron shell has its complement of two electrons, the next six electrons will find a home in one of the p orbitals.
Electron configuration for Molybdenum (element 42 ... Orbital diagram. Molybdenum electron configuration ← Electronic configurations of elements . Mo (Molybdenum) is an element with position number 42 in the periodic table . Located in the V period. Melting point: 2617 ℃. Density: 10.28 g/cm 3. The order of filling the orbitals with electrons in the Mo atom is an exception to the rule. Expected electronic configuration 1s 2 2s 2 2p 6 3s …
Orbital Diagrams and Electron Configuration - Basic ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
Orbital Diagram For Germanium - schematron.org Silicon") and Its Use the orbital diagram to find the third and eighth electrons. PROBLEM: Write a set of .Which ground-state atom has an electron configuration described by the following orbital diagram? A antimony B germanium C indium D lead E tin%(1).
Silicon Carbide | AMERICAN ELEMENTS Silicon (atomic symbol: Si, atomic number: 14) is a Block P, Group 14, Period 3 element with an atomic weight of 28.085. The number of electrons in each of Silicon's shells is 2, 8, 4 and its electron configuration is [Ne] 3s 2 3p 2. The silicon atom has a radius of 111 pm and a Van der Waals radius of 210 pm. Silicon was discovered and first ...
en.wikipedia.org › wiki › Solar_cellSolar cell - Wikipedia An amorphous silicon (a-Si) solar cell is made of non-crystalline or microcrystalline silicon. Amorphous silicon has a higher bandgap (1.7 eV) than crystalline silicon (c-Si) (1.1 eV), which means it absorbs the visible part of the solar spectrum more strongly than the higher power density infrared portion of the spectrum.
valenceelectrons.com › electron-configuration-ofCarbon(C) electron configuration and orbital diagram Carbon(C) is the 6th element in the periodic table and its symbol is ‘C’. This article gives an idea about the electron configuration of carbon and the orbital diagram, period and groups, valency and valence electrons of carbon, bond formation, compound formation, application of different principles.
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
iperiodictable.com › nitrogen-electron-configurationOrbital Diagram For Nitrogen (N) | Nitrogen Electron ... Ground State Electron Configuration For Nitrogen. When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s 2 2s 2 2p 3.Below you can get the full image representation which will help you to understand the topic more easily.
Electron configuration of silicon (Si), orbital diagram ... Orbital Diagram, electron configuration, and the noble gas notation for a silicon (Si) atom.
Carbon Bohr Model - How to draw Bohr diagram for Carbon(C ... S orbital can hold maximum of 2 electrons and P orbital can hold maximum of 6 electrons. FAQ. How many electron shells a Carbon Bohr model contains? Electron shell also called energy level, you can find the number of electron shells for an element by knowing its period number in the periodic table. The elements or atoms in the first period of the periodic table have one energy …





















0 Response to "39 Orbital Diagram Of Silicon"
Post a Comment