40 Cf Molecular Orbital Diagram
Molecular Orbital diagram of NO(nitric oxide) molecule Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12. Hybridization 4 - Lecture notes 1 - MOLECULAR ORBITAL DIAGRAM... Molecular orbital diagram key. Draw molecular orbital diagrams for each of the following molecules or ions. Determine the bond order of each and use this to predict the stability of the bond.
8.4 Molecular Orbital Theory - Chemistry The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Figure 10. The molecular orbital energy diagram predicts that He2 will not be a stable molecule, since it has equal numbers of bonding and antibonding electrons.
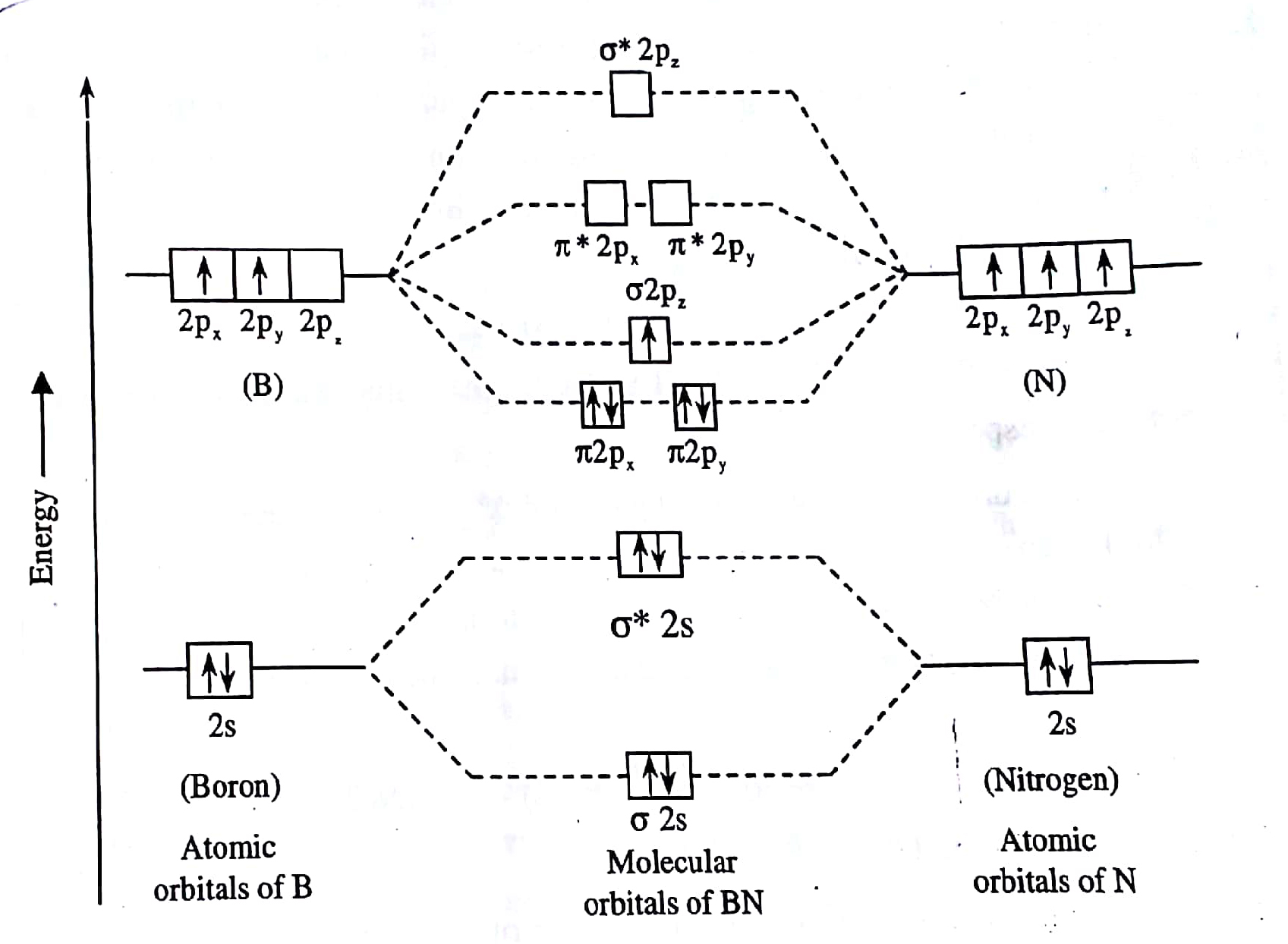
Cf molecular orbital diagram
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here. However, with more atoms, computers are required to calculate how the atomic orbitals combine. See three-dimensional drawings of the... Asked for: molecular orbital energy-level diagram, valence electron... A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. We can therefore use a molecular orbital energy-level diagram and the calculated bond order to predict the relative stability of species such as H2+. What is the molecular orbital energy diagram of CO? - Quora A molecular orbital diagram , or MO diagram , is a qualitative descriptive tool explaining chemical bonding. The electronic of hydrogen and fluorine are 1s¹ and 1s²2s²2p⁵ respectively. In the formation of HF molecule ,only 2p electrons of fluorine atom would combine effectively with the solitary electron...
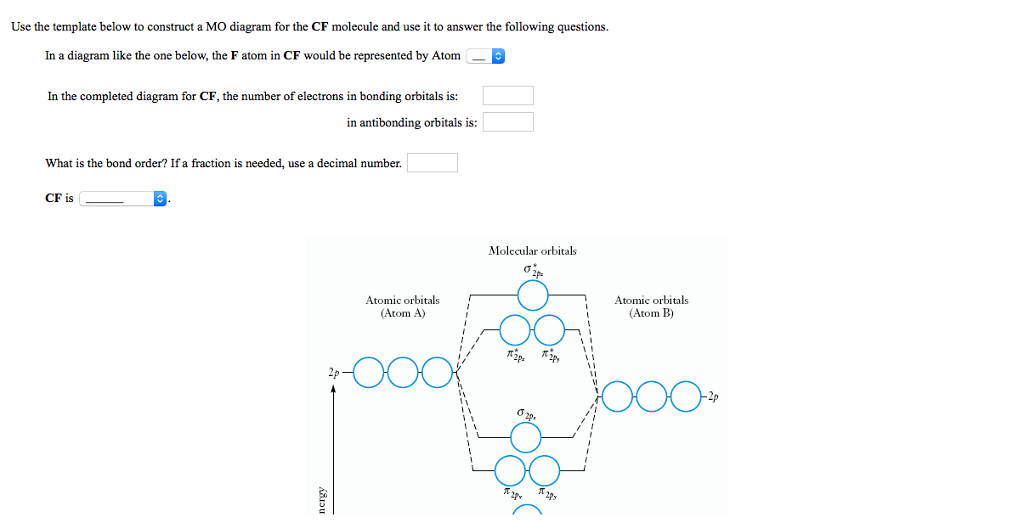
Cf molecular orbital diagram. Molecular orbital diagrams - Overleaf, Online LaTeX Editor Molecular orbital diagrams provide qualitative information about the structure and stability of the electrons in a molecule. This article explains how to create molecular orbital diagrams in LaTeX by means of the package MOdiagram. For information about the more traditional molecular structure... Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the... PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... • Molecule orbital theory (Robert Mullikan). • Electrons are delocalised - Different to Lewis and hybridisation (these are not MO). • Energy level diagram represents this interaction. - Two s orbitals interaction to create a low energy bonding and high energy anti-bonding molecular orbital. SOLVED:Construct the molecular orbital diagram fo Problem 39 Medium Difficulty. Construct the molecular orbital diagram for CF. Would you expect the bond length of $\mathrm{CF}^{+}$ to be longer or shorter than that of CF? To both CF and CF' ions, CF' has shorter bond length than CF. This is because CF' bond order more than CF molecule.
Molecular Orbital Theory: Explanation, Illustrations and... - Embibe Molecular Orbital Theory: To simplify things, we will consider the interaction of the orbitals containing valence electrons to create molecular orbitals. Have you ever thought about how sigma and pi bonds are formed? What is the difference between diamagnetic and paramagnetic behaviour? Molecular Orbital Diagrams -- Chemistry X - YouTube For chem videos, quizzes and more download Chemistry X for free on the App Store! Correlation Diagrams - by considering the positions and energies of... Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories If you can understand the foundation and skeleton of the diagram specific to that molecule, then it will be easier and faster for you to draw it. FIG. 1. Molecular orbital diagrams for the valence shells of (a) CF 4... ... corresponding molecular orbital diagrams, depicting the dominant atomic orbital characters of the occupied and some of the low-lying unoccupied In particular, the GOS profile of the C 1s→σC-Cl∗ [lowest unoccupied molecular orbital (LUMO)] transition of CF3Cl was found to contain relatively...
Wikizero - Molecular orbital diagram A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.[1][2][3] A fundamental principle of these theories is that... PDF Molecular | 90" (porbitals). This dilemma has been resolved by orbital Notes on. Molecular Orbital ~ a l c u l aitons. Notes on molecular orbital calculations. First printing, 1961 Second printing, with corrections, 1962 Third 'Cf. C. A. Coulson, Q u a r t e r l y Reviews, 144 (1947). 2 ~ P.auling, "Nature of the Chemical Bond, " pp. 14-15... MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams Accordingly, a molecular orbital diagram such as Figure 9-5 is inappropriate for heteronuclear diatomic molecules. If the two elements are similar (as in NO or CN mole-cules, for example), we can modify the diagram of Figure 9-5 by skewing it slightly.
Molecular Orbital Diagrams - Every Science The procedure for working out a molecular orbital of a general diatomic molecule is quite simple. We construct molecular orbitals using the available orbitals on the atoms. We then fill up the molecular orbitals, starting with the lowest in energy, until all the electrons in the species have been assigned to...
Energy level diagram for Molecular orbitals - Chemical Bonding and... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons. 2) If Nb < Na , the molecule is unstable because the antibonding influence is greater than the bonding influence, resulting in net force of repulsion.
Molecular orbitals editor | Chemissian: software to analyze spectra... Summary Molecular orbitals editor allows to build, analyze and graphically edit molecular/Kohn-Sham orbitals diagrams from the results of for analysis/editing, e.g. by selecting Orbital/Peak Selection tool and clicking on the molecular orbital energy level of interest you will see in AO Coefficients window...
The Pi Molecular Orbitals of Butadiene And How To Draw Them The Lowest-Energy Molecular Orbital (π1) Of The Butadiene Pi System Has Zero Nodes The Full Molecular Orbital Diagram For The Butadienyl System (n=4) A molecular orbital diagram without electrons is like an apartment building without people.
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep The molecular orbital diagram representing this order of energy levels is shown in fig. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if the value is negative or zero, it means that the molecule is unstable.
Molecular Orbital Theory Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the The bonding molecular orbital concentrates electrons in the region directly between the two nuclei. The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both...
PDF Chapter 5 | 5.2.2 Orbital Mixing Molecular orbital theory uses group theory to describe the bonding in molecules; it comple-ments and extends the introductory bonding models in Chapter 3 . In molecular orbital theory the symmetry properties and relative energies of atomic orbitals determine how these orbitals interact to form...
Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
Molecular Orbital Theory: Energy level diagram for molecular orbitals Molecular orbital theory was put forward by Hund and Mullikan in 1932. This theory is modern and more rational. This theory assume that in molecules, atomic orbitals lose their identity and the electrons in molecules are present in new orbitals called molecular orbitals.
An introduction to molecular orbital theory The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive...
What is the molecular orbital energy diagram of CO? - Quora A molecular orbital diagram , or MO diagram , is a qualitative descriptive tool explaining chemical bonding. The electronic of hydrogen and fluorine are 1s¹ and 1s²2s²2p⁵ respectively. In the formation of HF molecule ,only 2p electrons of fluorine atom would combine effectively with the solitary electron...
Asked for: molecular orbital energy-level diagram, valence electron... A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. We can therefore use a molecular orbital energy-level diagram and the calculated bond order to predict the relative stability of species such as H2+.
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here. However, with more atoms, computers are required to calculate how the atomic orbitals combine. See three-dimensional drawings of the...














![SOLVED:Ia) Draw the Lewis structure for CH;CF, [4 pts] 2a ...](https://cdn.numerade.com/ask_images/9c158bbcac1e4903921204419982a7aa.jpg)
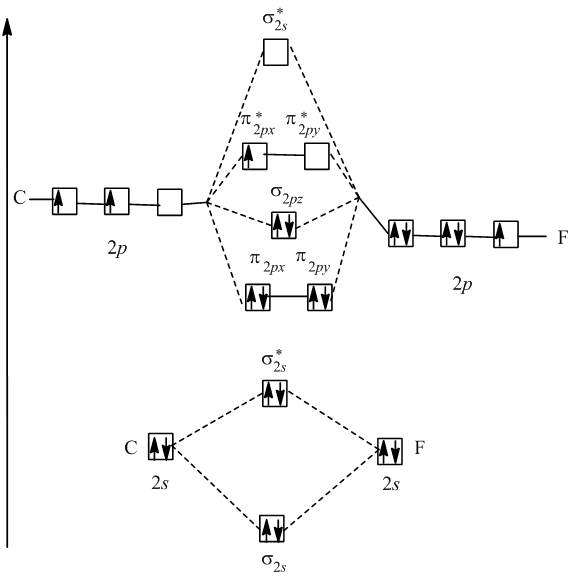
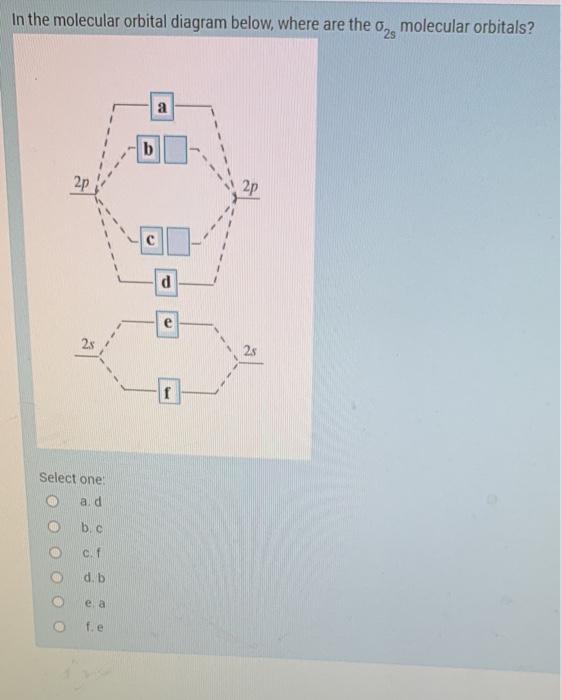





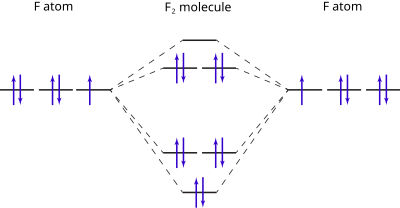



0 Response to "40 Cf Molecular Orbital Diagram"
Post a Comment