40 Lih Molecular Orbital Diagram
Simulating molecules(LiH) using VQE | by Rodney David | Medium LiH is a 12 body molecule containing 4 protons, 4 electrons, and 4 neutrons. This creates a 12 body model, which becomes intractable when simulating it both with a classical and quantum computer. ... We also set up the molecular orbitals to be considered and can reduce the problem size when we map to the qubit Hamiltonian. Hence, the amount of ... Molecular Orbitals - Molecular Orbitals for Heteronuclear ... Molecular Orbitals for Heteronuclear Molecules. The molecular orbitals which describe the motion of a single electron in a molecule containing two unequal nuclear charges will not exhibit the g and u symmetry properties of the homonuclear diatomic case. The molecular orbitals in the heteronuclear case will in general be concentrated more around one nucleus than the other.
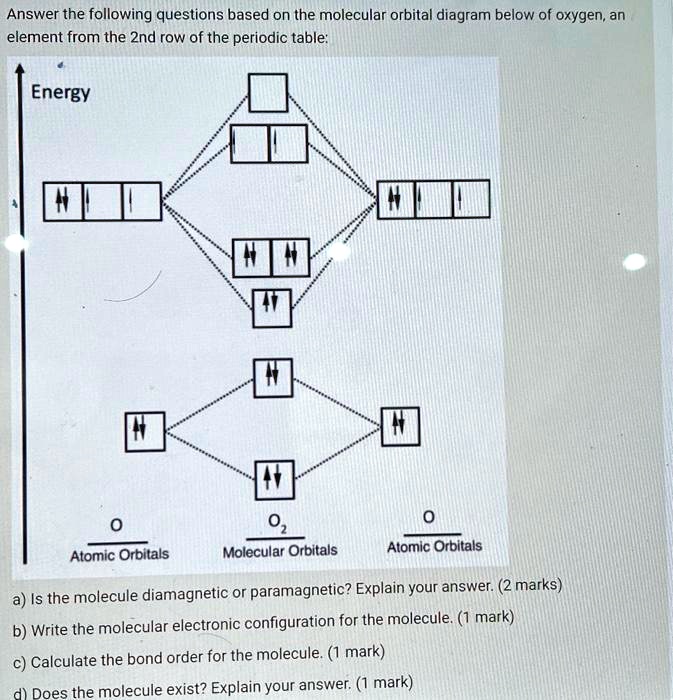
Draw a semi-quantitative molecular orbital diagram for LiH ... BHF molecular orbital diagram will look probably something like this where we have the one s electron in a hydrogen. And then we have the seven avails electrons available in Florence. Five of them being in the to P orbital's. The two s will probably just stay in the two s, its lowest energy. But then the floor een which is more electro negative.

Lih molecular orbital diagram
Split orbitals for the LiH molecule | SpringerLink The four-electron problem in LiH has been treated by use of Slater type orbitals for the 1s electrons on the Li atom and split molecular orbitals for the two valence electrons. Some properties of the two dimensional spin space present in the case of four different space-functions are discussed. Total electronic energies and electric dipole moments have been calculated. A Revisit to Molecular Orbitals in H2+, LiH, HF, and ... It is considered that the usual explanations for the molecular orbitals of H-2(+), LiH, HF, and hybridization in the conventional textbooks are liable to misunderstanding. Discover the world's ... PDF CHEM3023: Spins, Atoms and Molecules Molecular orbitals •A wavefunction for a single electron is called a molecular orbital (MO) ... •For example, either of the following choices for the two orbitals of the LiH molecule can be used to construct acceptable Slater determinants CHEM3023 Spins, Atoms and Molecules
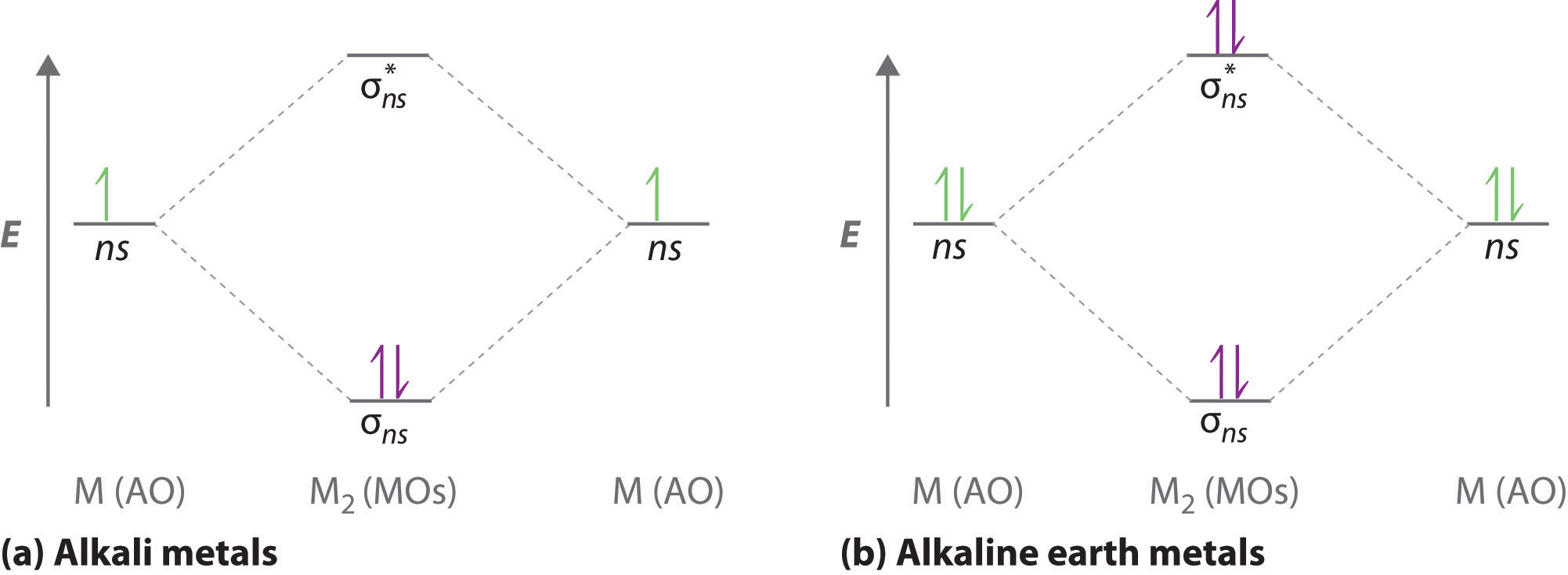
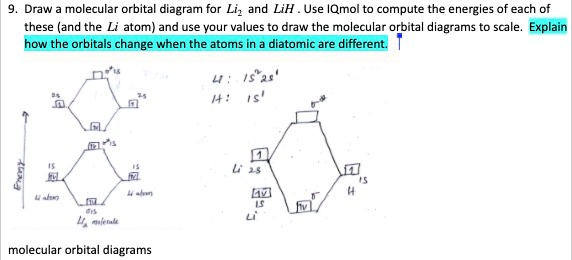
Lih molecular orbital diagram. LiH Molecular Orbital Diagram - why is the H1s AO lower ... LiH Molecular Orbital Diagram - why is the H1s AO lower than Li2s AO when Li2s has higher Zeff? 7 comments. share. save. hide. report. 100% Upvoted. This thread is archived. New comments cannot be posted and votes cannot be cast. Sort by. best. level 1. 1 year ago. PDF A Molecular Orbital Study of the Dipole Moment of HF, LiH ... In the ab initio molecular orbital calculations, the widely used 6-311G** basis sets are adopted throughout this study. 7,8 We use the MCSCF wave functions in order to treat the dissociation limit correctly. We use CASSCF within the configuration of 2 electrons in 2 orbitals for LiH, full-CI for Properties of water - Wikipedia Water has a very high specific heat capacity of 4184 J/(kg·K) at 25 °C – the second-highest among all the heteroatomic species (after ammonia), as well as a high heat of vaporization (40.65 kJ/mol or 2257 kJ/kg at the normal boiling point), both of which are a result of the extensive hydrogen bonding between its molecules. These two unusual properties allow water to … Dilithium - Wikipedia Dilithium, Li 2, is a strongly electrophilic, diatomic molecule comprising two lithium atoms covalently bonded together. Li 2 is known in the gas phase.It has a bond order of 1, an internuclear separation of 267.3 pm and a bond energy of 102 kJ/mol or 1.06eV in each bond. The electron configuration of Li 2 may be written as σ 2.. It has been observed that 1% (by mass) of …
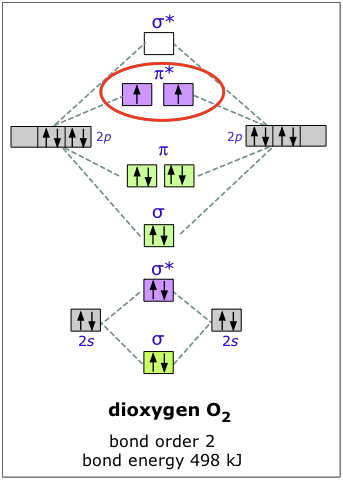
The Journal of Physical Chemistry C | Vol 126, No 5 10.2.2022 · Energy and Catalysis in China Virtual Special Issue. Collage of artwork from some of the papers included in the Energy and Catalysis in China Virtual Special Issue. Top left: Oxygen Groups Enhancing the Mechanism of Nitrogen Reduction Reaction Properties on Ru- or Fe-Supported Nb2C MXene (J. Phys. Chem. C 2021, 125 (27), 14636–14645. DOI: … 3.3.4: Assembling a complete MO diagram - Chemistry LibreTexts Exercise 3.3.4. 3. Construct a qualitative molecular orbital diagram for chlorine, Cl 2. Compare the bond order to that seen in the Lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). Answer. PDF The Electronic Structure of Molecules - An Introduction to ... the direction of overlap of the p orbitals on the two atoms, Figure 2. Sigma bonds are usually stronger because the atomic p-orbitals point towards each other and have better overlap. For double bonds, the first bond is sigma type and the second bond is pi-type. When two atomic orbitals overlap, two molecular orbitals are formed. One of the Institute Of Infectious Disease and Molecular Medicine For information on South Africa's response to COVID-19 please visit the COVID-19 Corona Virus South African Resource Portal.
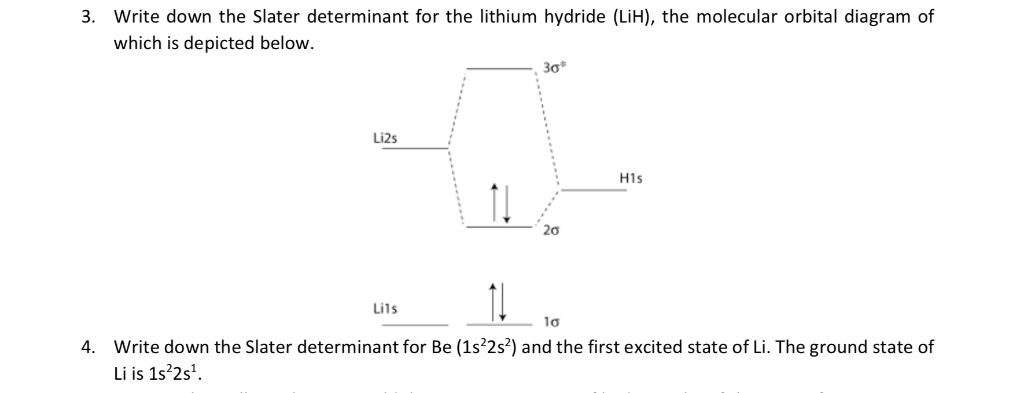
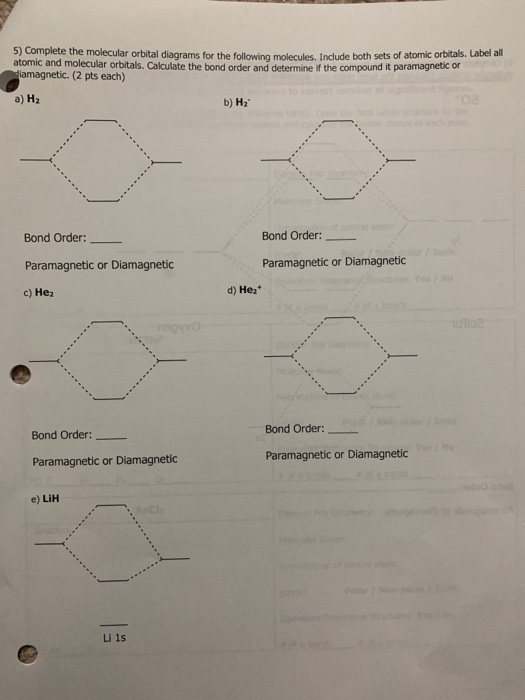
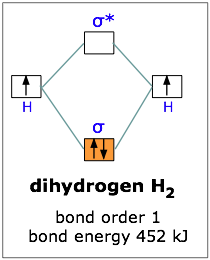
Draw a semi-quantitative molecular orbital diagram for LiH. Draw a semi-quantitative molecular orbital diagram for LiH. Subject: Chemistry Price: Bought 3 Share With. Draw a semi-quantitative molecular orbital diagram for LiH. Label the LUMO and HOMO. PDF Molecular orbital DiagraM - Magadh University Molecular orbitals: Orbitals that span two or more atoms. These are constructed by overlapping atomic orbitals (AOs) which match in symmetry and size. In principle, To construct MO diagram of a any Molecule, first, set up Schrödinger wave equation for that molecule and then, solve it!!! Solved 3. Write down the Slater determinant for the ... Write down the Slater determinant for the lithium hydride (LiH), the molecular orbital diagram of which is depicted below. Li2s H1s 20 Lils lơ Write down the Slater determinant for Be (1s22s2) and the first excited state of Li. The ground state of Li is 1s 2s1 4. PDF Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
Molecular Orbitals - Chem1 The lithium 1sorbital is the lowest-energy orbital on the diagram. Because this orbital is so small and retains its electrons so tightly, it does not contribute to bonding; we need consider only the 2sorbital of lithium which combines with the 1sorbital of hydrogen to form the usual pair of sigma bonding and antibonding orbitals.
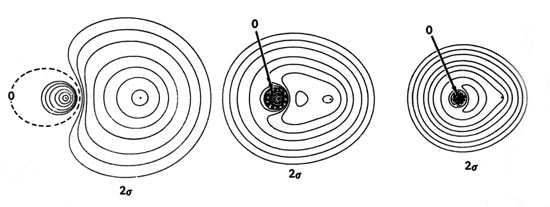
A Molecular Orbital Energy Level Diagram of LiH We show an molecular orbital energy level diagram of LiH obtained by ab initioHartree-Fock SCF-MO calculation with 6-311++G** basis set in this note. The 2σlevel of LiH is drawn at a higher position than the 1s of H in this diagram. The 1s electron of H is thus destabilized in LiH.
A Revisit to Molecular Orbitals in H2+, LiH, HF, and ... We have drawn three-dimensional contour plots of H2+ molecular orbitals and carbon hybrid orbitals according to explanations given in quantum chemistry textbooks and have also performed ab initio molecular orbital calculations of LiH and HF. Some contour representations and molecular orbital energy-level diagrams thus obtained are not consistent with the figures adopted in the textbooks. It is ...
PDF Valence-only correlation in LiH and BeH+ - NIST coefficients are given in table 2. The first two orbitals are exactly the undisturbed Isu H. F. molecular orbital and the bonding PNO which is very similar to the second occupied H.F. molecular orbital. This orbital has been referred to as a ISH _ orbital because of the ionicity of the LiH bond [8]. However, the 2sLi and
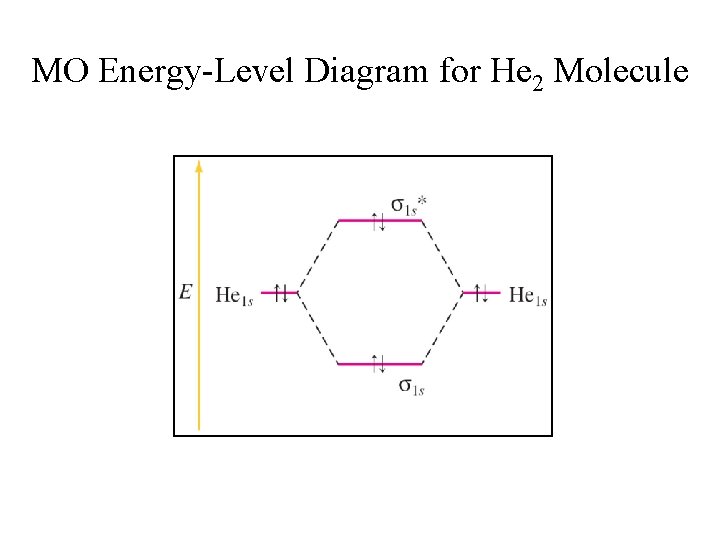
Molecular Orbital Diagram Of Lih Molecular Orbital Diagram Of Lih Siagram MO diagram for dihelium looks very similar to that of dihydrogen, but each helium has two electrons in its 1s atomic orbital rather than one for hydrogen, so there are now four electrons to place in the newly formed molecular orbitals. Wiring Diagrams Free DOWNLOAD Molecular Orbital Diagram Of Lih
Clayden J. - Organic Chemistry - PDF Free Download The molecular ion in the E.I. mass spectrum of the bromo-amide below has two peaks at 213 and 215 of roughly equal intensity. This might just represent the loss of molecular hydrogen from a molecular ion 215, but, when we notice that the first fragment (and base peak) has the same pattern at 171/173, the presence of bromine is a more likely explanation.
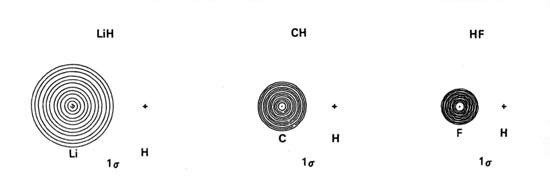
Molecular Orbital Diagram Of Lih - schematron.org Molecular Orbital Diagram Of Lih. We shall consider the molecular orbitals in LiH, CH and HF to illustrate how molecular orbital theory describes the bonding in heteronuclear molecules, and to. and 2p orbitals, but that is not how sodium chloride is made. Sodium atoms are Construct an MO diagram for LiH and suggest what type of bond it might have.
General Chemistry Questions a. it has such a low molecular weight. b. it is rather dense. c. the O-H single bond has a high bond energy. d. it has many relatively strong hydrogen bonds. e. it dissolves both ionic and covalent compounds. 8. The triple point is a. an end to the liquid-gas line in a phase diagram. b.
Solved QUESTION 32 Part B_Question : Draw a | Chegg.com QUESTION 32 Part B_Question : Draw a semi-quantitative molecular orbital diagram for LiH. Label the LUMO and HOMO. Attach File Browse My Computer Browse Content Collection QUESTION 33 Part B_Question : Sketch the following complexes. If more than one enantiomer exists, sketch just one.
Organic Chemistry 4th ed - Paula Bruice - Academia.edu Organic Chemistry 4th ed - Paula Bruice
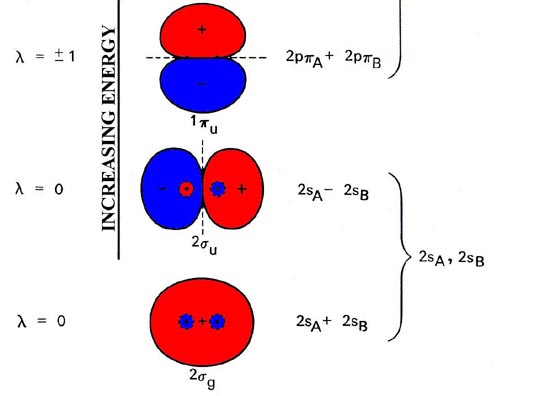
PDF Using Symmetry to Generate Molecular Orbital Diagrams atomic orbitals a 1g (σ g) 2s e 1u (π u) p y p x p z a 1u (σ u) atomic orbitals of terminal atoms H 1 + H 2 LGO (1) LGO (2) a 1u (σ u) …..thus, the symmetry of LGO(2) matches that of the X atom's p z - orbital …..and LGO(2) will combine with the X p z -orbital to form a new molecular orbital (MO) …..this interaction is symmetry ...
ap chem semester 1 MCQs Flashcards - Quizlet Start studying ap chem semester 1 MCQs. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Molecular Orbitals Calculation on LiH with Algebraic ... Molecular Orbitals Calculation on LiH with Algebraic Treatment of the Integrals. In this work we employ an algebraic computational method to solve the integrals which arise in the study of diatomic molecules. Using the Slater-type orbitals (STO), we obtain analytical solutions for the one-center and two-center hybrid and Coulomb integrals.
bond - The molecular orbitals of lithium hydride ... The occupied atomic orbitals of lithium are 1s and 2s, with energies of around -66 eV and − 5.3 e V respectively. The occupied atomic orbital of hydrogen is 1s, with an energy of -13.6 eV.
Organic Chemistry 10th (Solomon and Fhryle) - Academia.edu Enter the email address you signed up with and we'll email you a reset link.
2.5.5: Molecular Orbital Diagrams - Chemistry LibreTexts The lithium 1 s orbital is the lowest-energy orbital on the diagram. Because this orbital is so small and retains its electrons so tightly, it does not contribute to bonding; we need consider only the 2 s orbital of lithium which combines with the 1 s orbital of hydrogen to form the usual pair of sigma bonding and antibonding orbitals.
Density Functional Theory Study of Metallic Silicon (111 ... 2.2.2022 · The band structure on the surface might be influenced by the abruptly ended periodic structure and change the physical properties of the semiconductor. By using the density functional theory, this research also demonstrates that the Si unit cell has the calculated room-temperature electrical conductivity as 4.01 × 10–6 (Ω–1 cm–1), similar to the experimental result. Thus, the …
PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
LiH - MO Diagram - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Hybridization - TU Braunschweig Hybrization. Now we want to consider the example of Lithiumhydride LiH and apply the LCAO description of molecular orbitals. Ab initio calculations (derived from the self consistent field approach) with a minimal basis (i.e. the Li atom with 1s-, 2s- and 2p-atomic orbitals and Φ 1s, Φ 2s and Φ 2p as wave function and a hydrogen wave function Φ H) produce (neglecting a minor involvement of ...
A Molecular Orbital Energy Level Diagram of LiH | Request PDF It is considered that the usual explanations for the molecular orbitals of H-2(+), LiH, HF, and hybridization in the conventional textbooks are liable to misunderstanding. View.
PDF CHEM3023: Spins, Atoms and Molecules Molecular orbitals •A wavefunction for a single electron is called a molecular orbital (MO) ... •For example, either of the following choices for the two orbitals of the LiH molecule can be used to construct acceptable Slater determinants CHEM3023 Spins, Atoms and Molecules
A Revisit to Molecular Orbitals in H2+, LiH, HF, and ... It is considered that the usual explanations for the molecular orbitals of H-2(+), LiH, HF, and hybridization in the conventional textbooks are liable to misunderstanding. Discover the world's ...
Split orbitals for the LiH molecule | SpringerLink The four-electron problem in LiH has been treated by use of Slater type orbitals for the 1s electrons on the Li atom and split molecular orbitals for the two valence electrons. Some properties of the two dimensional spin space present in the case of four different space-functions are discussed. Total electronic energies and electric dipole moments have been calculated.
















0 Response to "40 Lih Molecular Orbital Diagram"
Post a Comment