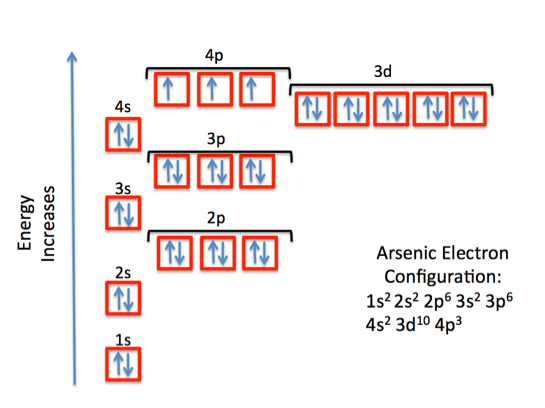
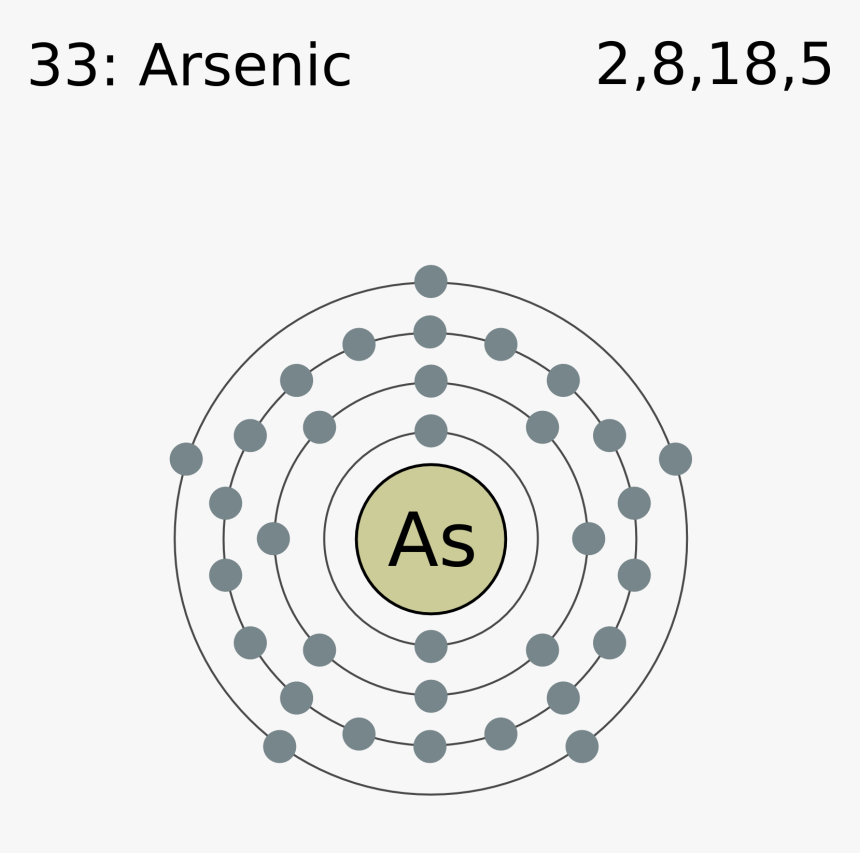
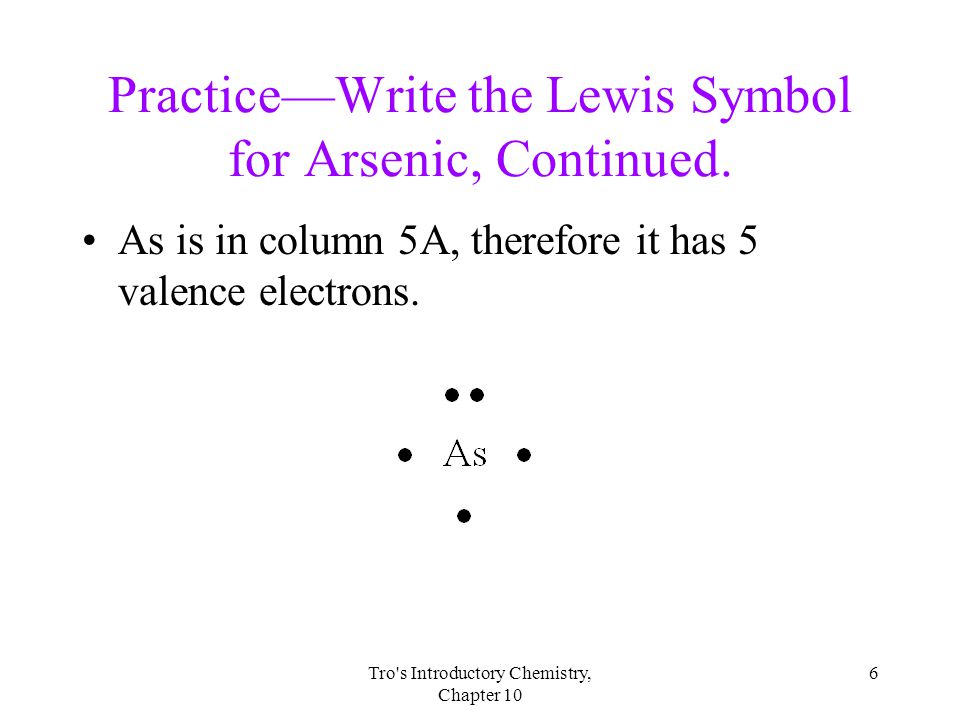
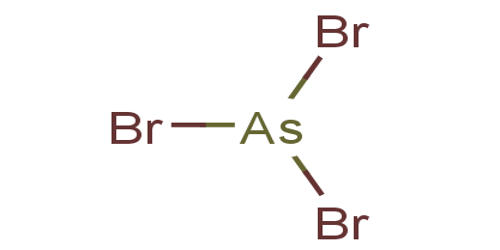
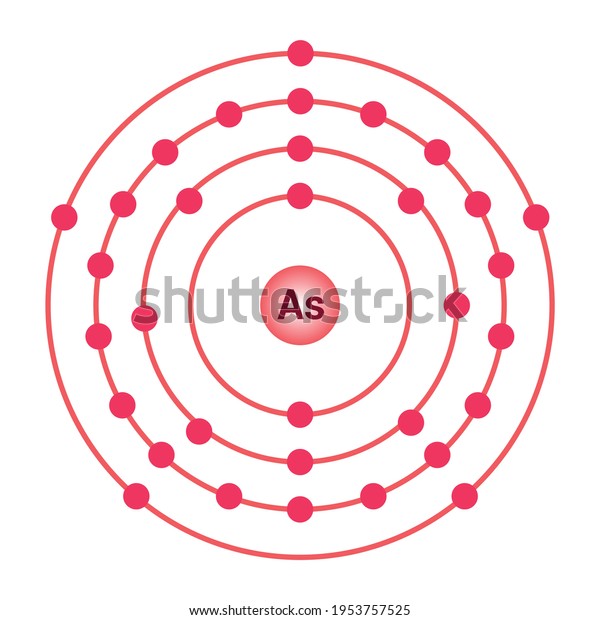
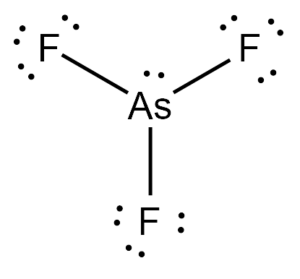
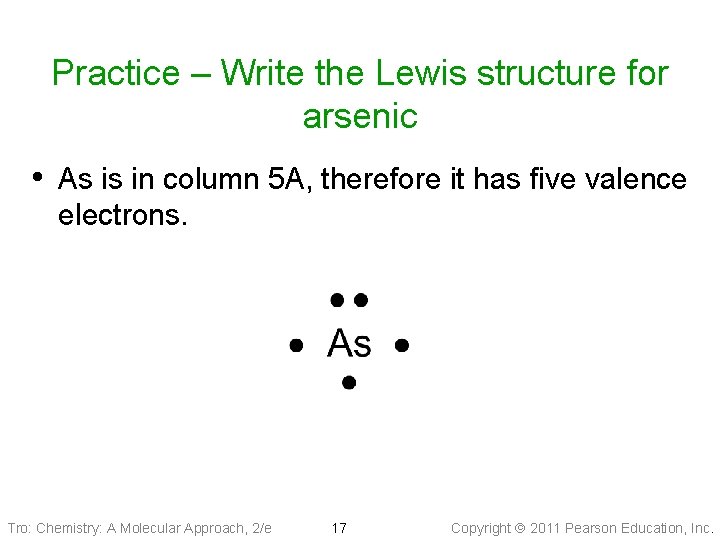
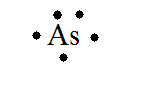
42 arsenic electron dot diagram
The non-appearance of the electron in the covalent bond can be represented by a small circle. Therefore Hole can be shown as an empty place or vacancy left behind in the crystal structure. Since an electron is having unit negative charge, the hole will be similarly having a unit positive charge. 2.2. Mechanism of conduction of Electrons and Holes: 21.08.2015 · The electron then dissipates its energy in the external circuit and returns to the solar cell. A variety of materials and processes can potentially satisfy the requirements for …
Academia.edu is a platform for academics to share research papers.
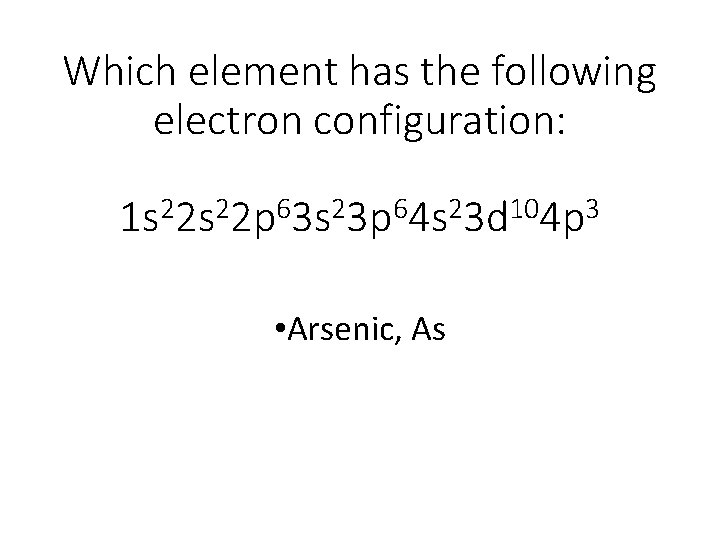
Arsenic electron dot diagram
Draw a ‘dot-and-cross’ diagram to show the bonding in an Al H 4 – ion. Show outer electrons only. [1] (b) Nitrogen forms NH 4 + and NH 2 – ions. Predict the name of the shape of, and H–N–H bond angle in, NH 4 + and NH 2 –. Ion Name of shape H–N–H bond angle NH 4 + Academia.edu is a platform for academics to share research papers. Get access to every article of chemistry with in-depth content and well-illustrated images which will help you understand all of the topics of chemistry for board exam as well as competitive exam preparation.
Arsenic electron dot diagram. (ii) Draw a dot‑and‑cross diagram to show the electron arrangement in a molecule of iodine monochloride. Show outer shell electrons only. [2] (e) Potassium bromide has a melting point of 734 °C. Iodine monochloride has a melting point of 27 °C. In terms of attractive forces, explain why there is a large difference between these melting ... Bromine (Br), Fluorine (F), Sulfur (S), Arsenic (As) 28. (15 points) Draw the Lewis structure of carbonic acid, H 2CO 3, and determine the formal charge of each atom in the structure. 29. (15 points) Draw the molecular orbital energy diagram for nitrogen monoxide, NO, and determine the bond order of the molecule. (Reminder: place the atomic ... 26.09.2021 · There is a deficit of one electron, and as a result, the fourth electron has no electron with which to bind. As a result, a void or hole is produced, and it becomes necessary to fill it. As a result, an electron in the outer orbit of a nearby atom has a chance to leap and fill the void. In this way, one electron is removed from the system ... Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Andrea C. Ferrari * a, Francesco Bonaccorso ab, Vladimir Fal'ko c, Konstantin S. Novoselov d, Stephan Roche ef, Peter Bøggild g, Stefano Borini h, Frank H. L. Koppens i, Vincenzo Palermo j, Nicola Pugno klm, José A. Garrido n, Roman Sordan o, Alberto Bianco p, …
Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link. The first element of the periodic table is hydrogen and its position at the beginning of the periodic table. Hydrogen is a s-block element. This article gives an idea about the electron configuration of hydrogen, period and groups, valency and valence electrons of hydrogen, bond formation, compound formation, application of different principles. (A) Schematic diagram of the surface state effect of nanowires under different light sources. Under the irradiation of 1310-, 1550-nm, and 2-μm laser and blackbody light source, the ability of surface states to capture photogenerated electrons is different, so the gain caused by the surface states are different, respectively, about 1500, 4000 ... Get access to every article of chemistry with in-depth content and well-illustrated images which will help you understand all of the topics of chemistry for board exam as well as competitive exam preparation.
Academia.edu is a platform for academics to share research papers. Draw a ‘dot-and-cross’ diagram to show the bonding in an Al H 4 – ion. Show outer electrons only. [1] (b) Nitrogen forms NH 4 + and NH 2 – ions. Predict the name of the shape of, and H–N–H bond angle in, NH 4 + and NH 2 –. Ion Name of shape H–N–H bond angle NH 4 +


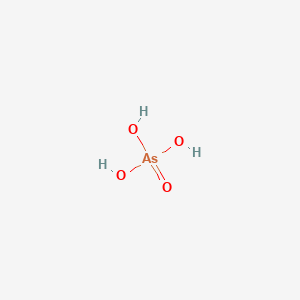
![Arsenic acid]](https://www.degruyter.com/document/doi/00.0000/IUPAC.iupac.compound.234/asset/images/234.png)


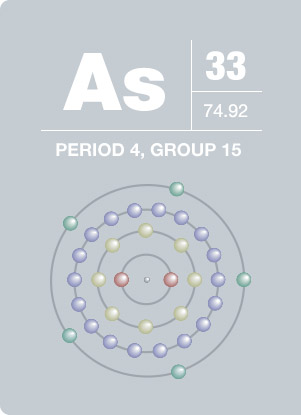








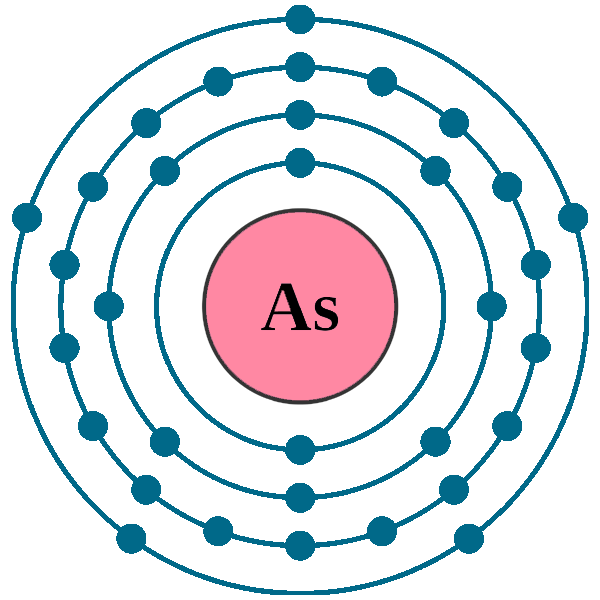



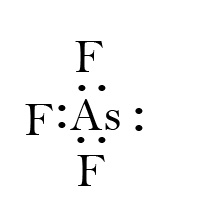





:max_bytes(150000):strip_icc()/Arsenic-58b602013df78cdcd83d2cac.jpg)
0 Response to "42 arsenic electron dot diagram"
Post a Comment