39 c22- molecular orbital diagram
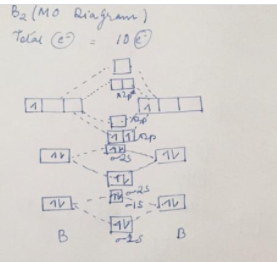
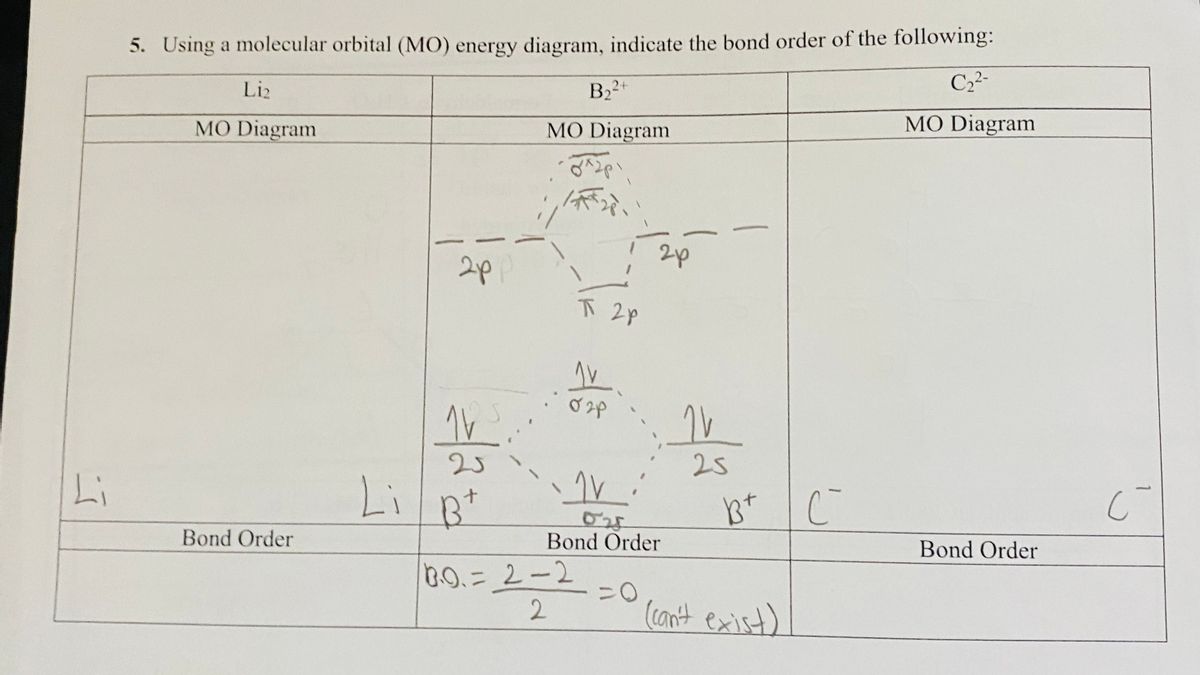
According to the molecular orbital theory, what is the ... "BO" = 1/2 Boron atom is atomic number 5 in the periodic table, so it has five electrons. Thus, B_2 carries ten total electrons. The atomic orbitals each boron contributes consists of the 1s, 2s, and 2p. The ns orbitals combine to give a portion of the molecular orbital (MO) diagram like this: where sigma^"*" indicates an antibonding sigma (sigma) MO, and sigma is the bonding MO. Arrange the following in order of decreasing stability. a ... Keywords: MO diagram, C22+, B22-, Li2-, molecular orbital diagram, paramagnetic, diamagnetic, paramagnetism, diamagnetism, paired electrons, unpaired electrons. Answer : For a species to be diamagnetic it needs to have no unpaired electrons and for paramagnetic the species needs t have unpaired electrons.
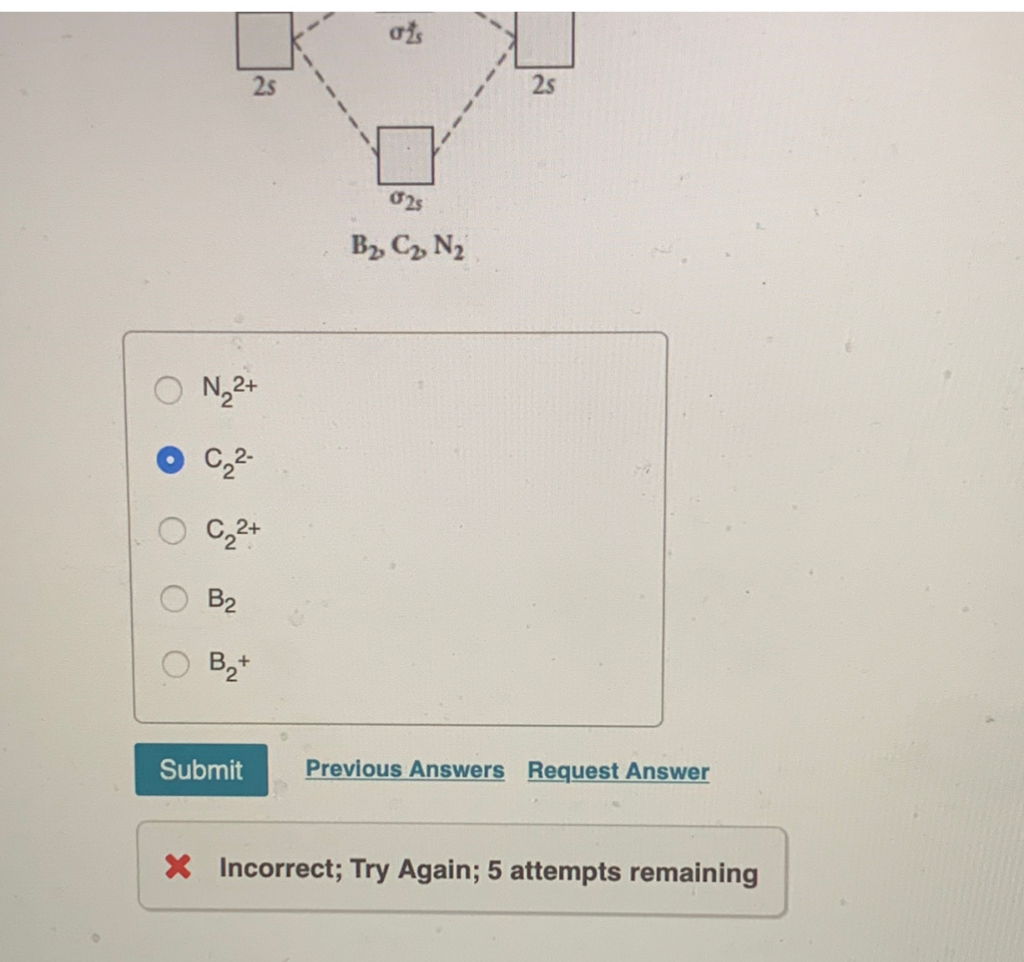
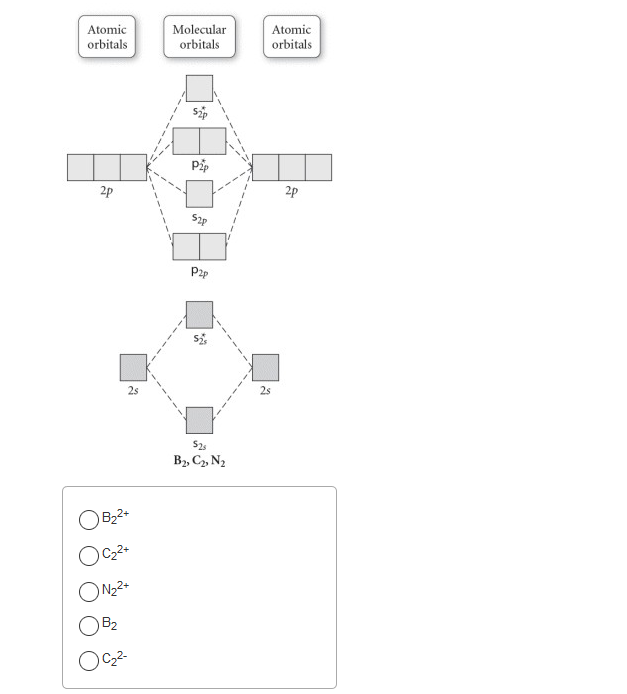
is b22+ paramagnetic or diamagnetic Whether a molecule is paramagnetic or not is only explained using molecular orbital theory. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.Answer options:B2B22+N22+C22-B22- Use the molecular orbital diagram shown to determine which of the following is most stable.
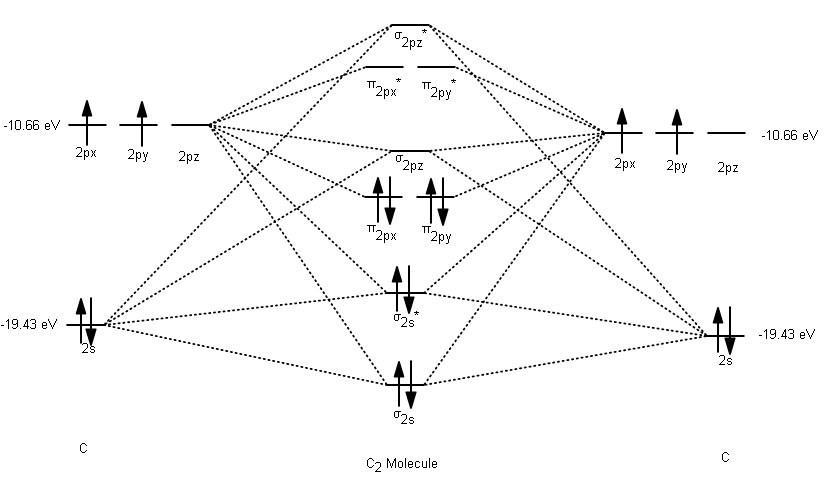
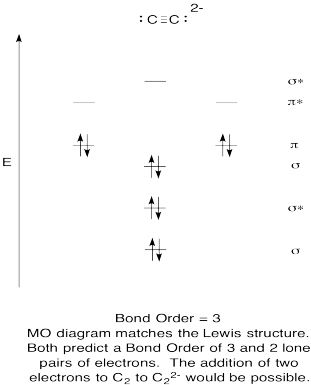
C22- molecular orbital diagram
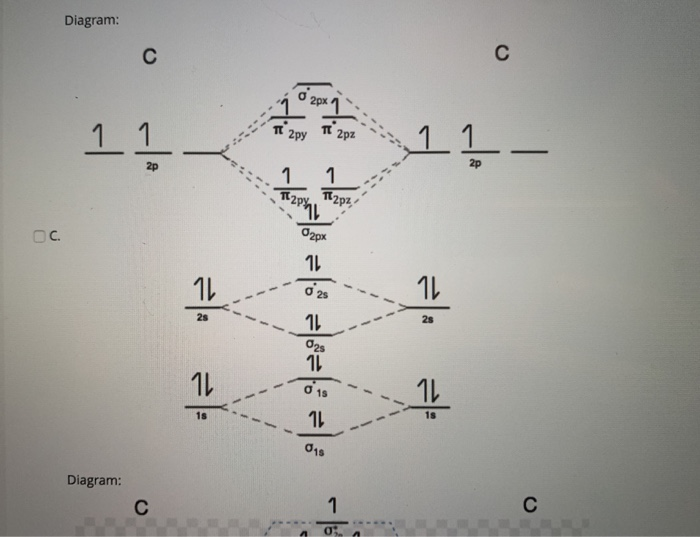
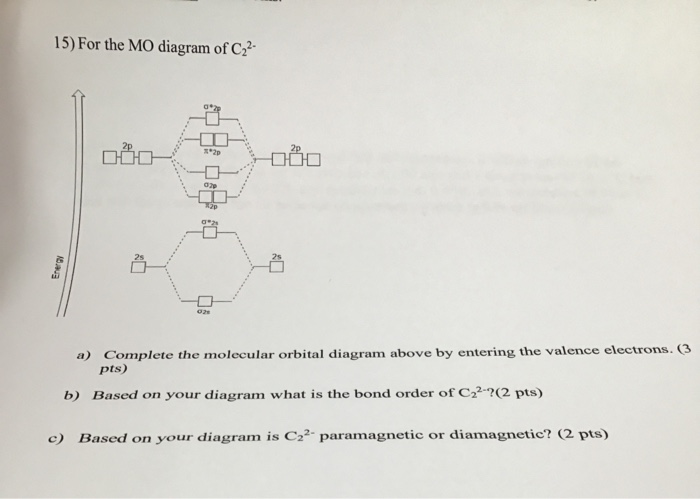
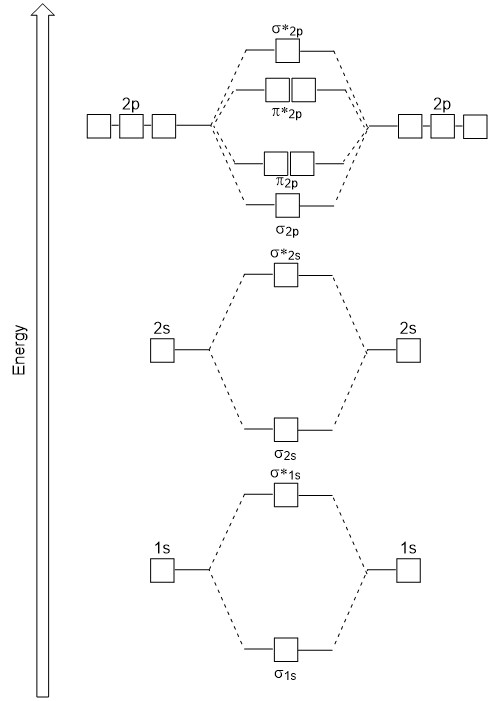
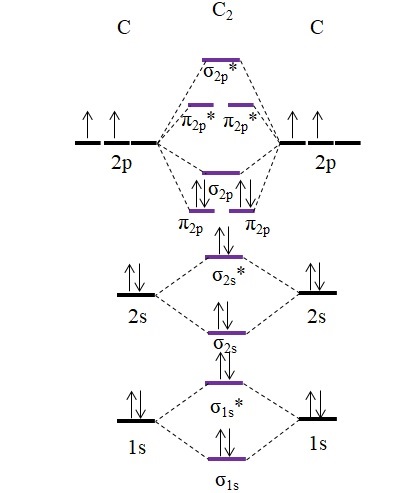
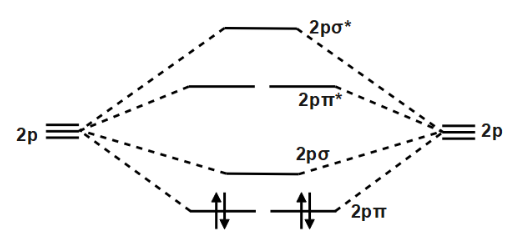
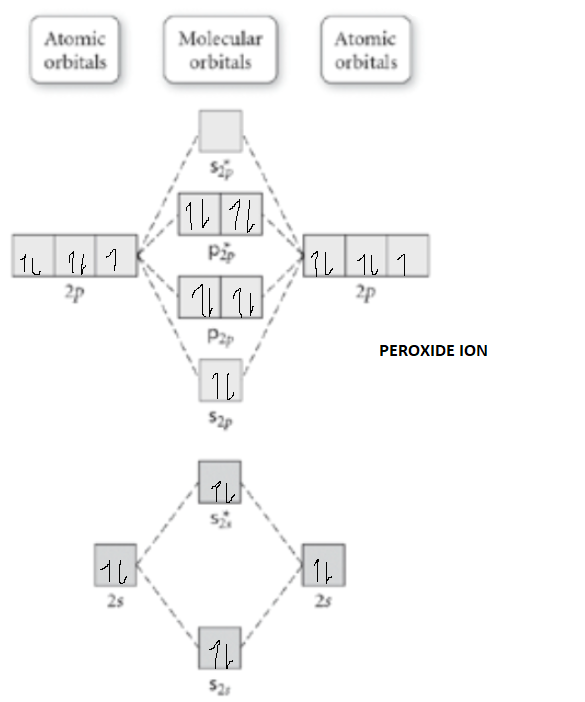
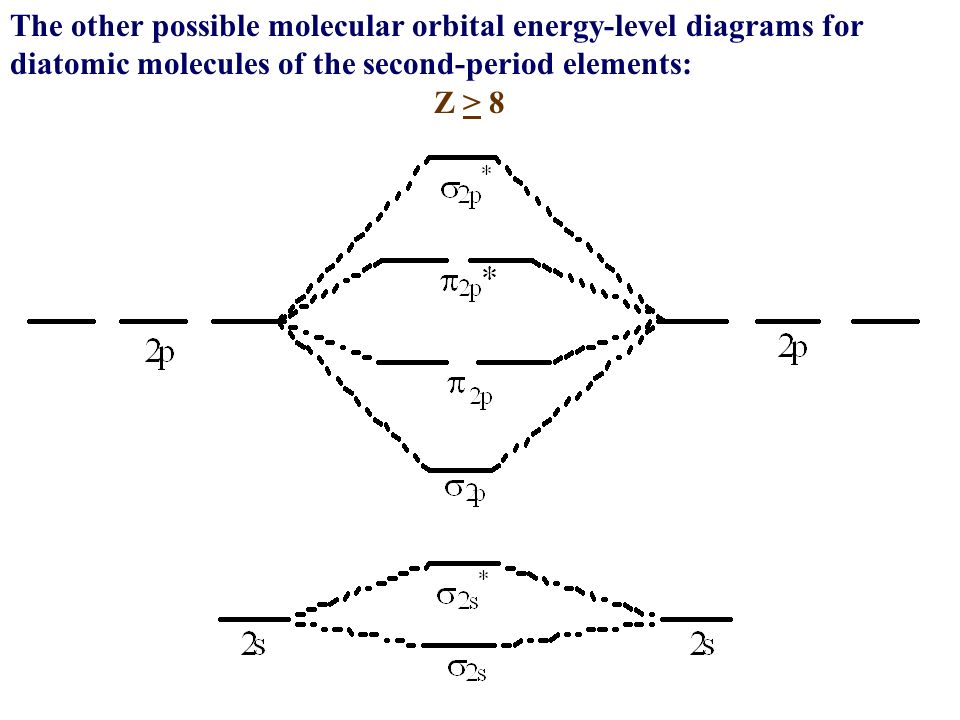
Use the molecular orbital diagram shown to determine which ... The given molecular orbital diagram contains orbitals to be filled in the order: s 1s, s* 1s, s 2s, s*2s, p 2p, s 2p, p*2p, s*2p According to molecular orbital theory, The electronic configuration of C 22- = s1s2, s*1s2, s2s2, s*2s2, p2p4, s2p2 => Number of unpaired electrons = 0 39 mo diagram of b2 - Wiring Diagram Images C22- Molecular Orbital Diagram B2 MO diagram with no sp mixing: B2 = 6 e⁻. Label the sigma bonding molecular orbitals on the diagram above using the designation. d. Give the electron configurations for the species C2 and C When we draw the C2 MO, we have everything up till the PiPy Orbitlal filled, and the next orbital tht would be filled ... Molecular Orbital (MO) Diagram for C2(2-) - YouTube When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i...
C22- molecular orbital diagram. MO Diagram for C2+ and C2- - CHEMISTRY COMMUNITY The answer is C2- because of bond orders. When we draw the C2 MO, we have everything up till the PiPy Orbitlal filled, and the next orbital tht would be filled would be the sigma2Pz orbital. As for bond orders it is 1/2* [ (#e- in bonding orbitals)- (#e- in antibonding orbitals)] Doing this, normally just C2 is 1/2* [ (8)-4]=2. Molecular Orbital Theory - Build C22+ - YouTube For the ion C22+:a) Draw the molecular orbital diagram.b) Calculate the bond order.c) Would this ion exist?d) Write the electron configuration of the ion PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in Answered: 4. Write a molecular orbital diagram,… | bartleby Solution for 4. Write a molecular orbital diagram, determine the bond order, and predict the magnetism for the followin C22- b. H22- а. c. Nez+ c. 022+
Li2 Mo Diagram - schematron.org Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Dilithium (Li2) The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in thebonding, but the 2s electrons fill thebonding orbital. Molecular orbital diagrams for HBr and HF. | Download ... The changes in Ni s, p , and d contributions to Ni-CO 4,5 bonding can be rationalized by contrasting the Ni s, p , and d molecular orbital displacements MOD's -the molecular analog of COD, for the ... Chapter 9 Molecular Orbitals in Chemical Bonding (Midterm ... molecular orbital diagram for F2. number of elections in the sigma*2p molecular orbital is. 0. the total numbers of electrons in the pi2p molecular orbital of B2 is. 2. the total numbers of electrons in the pi*2p molecular orbitals of F2 is. 4. what is the bond order for B2. 1. C22- Molecular Orbital Diagram - schematron.org C22- Molecular Orbital Diagram 07.02.2019 4 Comments The problem provides you with the MO diagram for the C2 molecule, so all you really have to do here is add an electron to that diagram. It is sigma2s (2)sigma2s* (2)sigma2p (2)pi2p (4)pi2p* (4) Bond order 1. It is stable. In fact, it's the perioxide ion. Check me out.
Chem Flashcards - Quizlet Use molecular orbital diagram shown to determine which is most stable a) O22-b)F2 c) F22+ d) F22-e) Ne22-a. Use the molecular orbital diagram shown to determine which of the following is most stable. A) F2 ... C22⁻ E) B22⁺ ... Solved Construct a molecular orbital diagram for C22 ... Construct a molecular orbital diagram for C22+. What would you predict for the bond order? Construct a similar diagram for this ion using the ordering for the heavier Period 2 elements and compare the two results. What experimental property could be used to confirm this different ordering? Question: Construct a molecular orbital diagram for C22 ... Molecular Orbitals: Problems and Solutions - SparkNotes By constructing a molecular orbital picture for each of the following molecules, determine whether it is paramagnetic or diamagnetic. a. B 2 b. C 2 c. O 2 d. NO e. CO a. B 2 is paramagnetic because it has two unpaired electrons, one in each of its p orbitals. b. C 2 is diamagnetic because all of its electrons are paired. Bond order of c2+ ,c2 2- - Brainly.in Bond order = 1/2 (number of electrons in bonding orbitals - number of electrons in antibonding orbitals) Therefore, Bond order of C2+ = 1/2 (5 - 2) = 3/2 = 1.5 Bond order of C2- = 1/2 (7 - 2) = 5/2 = 2.5 Bond order of C2 = 1/2 (6 - 2) = 2 Highest bond order means highest bond energy and shortest bond length.
37 use the molecular orbital diagram shown to determine ... Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.A) N22+ B) B2C) B22+D) C22-E) C22+ FREE Expert Solution Show answer Answer: Quiz 1 Flashcards | Quizlet Draw the molecular orbital diagram shown to determine which of the following is MOST stable A) B2^2+ B) C2^2+ C) N2^2+ D) C2^2-E) B2.
Use the molecular orbital diagram shown to determine which ... Keywords: MO diagram, C22+, B22-, Li2-, molecular orbital diagram, paramagnetic, diamagnetic, paramagnetism, diamagnetism, paired electrons, unpaired electrons. Answer : For a species to be diamagnetic it needs to have no unpaired electrons and for paramagnetic the species needs t have unpaired electrons.
PDF Practice Test Questions 3 Molecular Orbital Theory ... molecular orbitals. It is not necessary to draw pictures of the orbitals for this part of the question. (b) On your diagram, clearly label the HOMO(s) of 𝑁𝑁+. Draw a𝑂𝑂picture of this/these molecular orbital(s). Clearly indicate the phase, location of the nuclei, and relative amounts of electron density on each atom. ...
Solved Draw a molecular orbital diagram for F22- and use ... 1) F22- have one sigma bond, zero pi bond and the bond order is one. 2nd diagram is the correct molecular o …. View the full answer. Transcribed image text: Draw a molecular orbital diagram for F22- and use it to answer the questions below. Note that two half-filled pi orbitals give one pi bond.
Answered: Draw the molecular orbital diagram… | bartleby ASK AN EXPERT. ASK. Science Chemistry Q&A Library Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B22+, B2, C22-, B22-, and N22+. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B22+, B2, C22-, B22-, and N22+.
What is the molecular orbital diagram for C_2^-? | Socratic The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be.
C22- Molecular Orbital Diagram C22- Molecular Orbital Diagram The problem provides you with the MO diagram for the C2 molecule, so all you really have to do here is add an electron to that diagram. From my files: Explain the fact that the magnetic properties of B2 are consistent with the π2p.?
Molecular Orbital Theory - Purdue University Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Selected Answer d 8 Answers a 4 b 16 c 1 d 8 e none of ... Antibonding molecular orbitals have electron density mainly outside the space between the two nuclei. 3. All antibonding molecular orbitals are higher in energy than the atomic orbitals of which they are composed. Selected Answer: e. 2 and 3. ... C22 - e. B2 Question 27. 1 ...
PDF CH 222 Practice Problem Set #2 - MhChem 1. The electron-pair and molecular geometries are tetrahedral. The C atom is sp3 hybridized. Three of these hybrid orbitals each overlap with a chlorine 3p orbital to form three C—Cl sigma bonds. One hybrid orbital overlaps with a hydrogen 1s orbital to from a C—H sigma bond. 2. Answers: (a) BBr 3 trigonal planar trigonal planar sp2 (b) CO
Use the molecular orbital diagram shown to... | Clutch Prep Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A. Ne 22+ B. O 22+ C. F 22+ D. O 22- E. None of the above are paramagnetic. Learn this topic by watching MO Theory: Homonuclear Diatomic Molecules Concept Videos.
Molecular Orbital (MO) Diagram for C2(2-) - YouTube When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i...
39 mo diagram of b2 - Wiring Diagram Images C22- Molecular Orbital Diagram B2 MO diagram with no sp mixing: B2 = 6 e⁻. Label the sigma bonding molecular orbitals on the diagram above using the designation. d. Give the electron configurations for the species C2 and C When we draw the C2 MO, we have everything up till the PiPy Orbitlal filled, and the next orbital tht would be filled ...
Use the molecular orbital diagram shown to determine which ... The given molecular orbital diagram contains orbitals to be filled in the order: s 1s, s* 1s, s 2s, s*2s, p 2p, s 2p, p*2p, s*2p According to molecular orbital theory, The electronic configuration of C 22- = s1s2, s*1s2, s2s2, s*2s2, p2p4, s2p2 => Number of unpaired electrons = 0



















0 Response to "39 c22- molecular orbital diagram"
Post a Comment