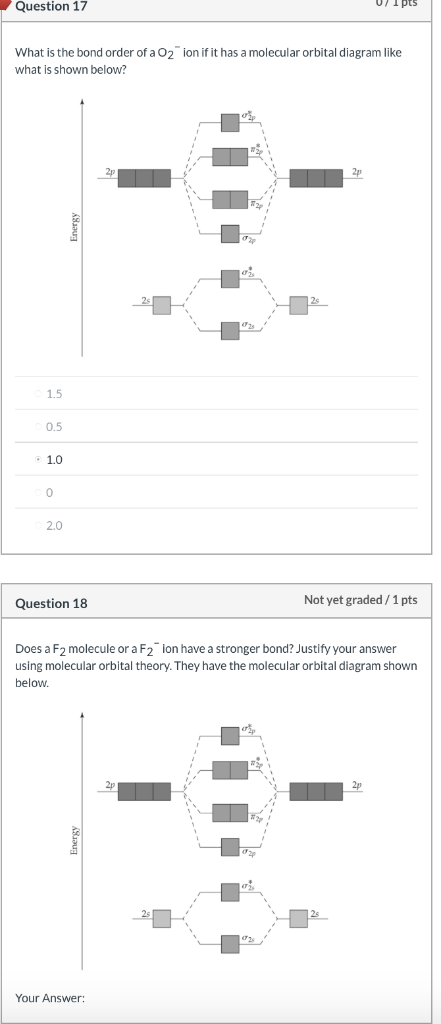
42 use the following mo diagram to find the bond order for o2.
Solved Use the MO diagram provided below to answer the ... Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 94% (16 ratings) Transcribed image text: Use the MO diagram provided below to answer the following questions: • What is the bond order for N? [Select] • Is N2 ... CN- lewis structure, molecular orbital diagram, and, bond ... Also, using the Molecular orbital diagram of CN-we can also find its bond order which helps us to predict its bond length and stability as well. Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2.
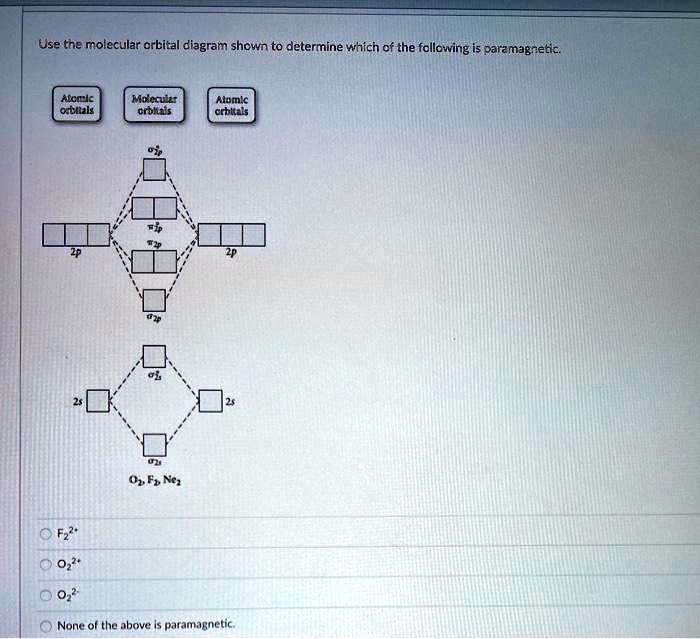
Molecular Orbital Theory - Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in .

Use the following mo diagram to find the bond order for o2.
Solved Use the MO diagram provided below to answer the ... Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (38 ratings) Transcribed image text: Use the MO diagram provided below to answer the following questions: • What is the bond order for C2?_ [Select] • Is C2 ... Draw the molecular orbital diagram of N2 and calculate the ... Draw the molecular orbital diagram of N2 and calculate the bond order. Answer: The bond order shows the number of chemical bonds present between a pair of atoms. The Bond Order Formula can be defined as half of the difference between the number of electrons in bonding orbitals and antibonding orbitals. Bond order formula is given as below. Solved re.com/courses/bb687qu7272es/65878 correct Question ... Without 2s-2p mixing With 2s-2p mixing CK :O r 25 20 2s AO MO AO AO MO AO A MO energy levels for O Fa. and Ne B MO energy levels for Ba Cz and Ng 0.5 This problem has been solved! See the answer See the answer See the answer done loading
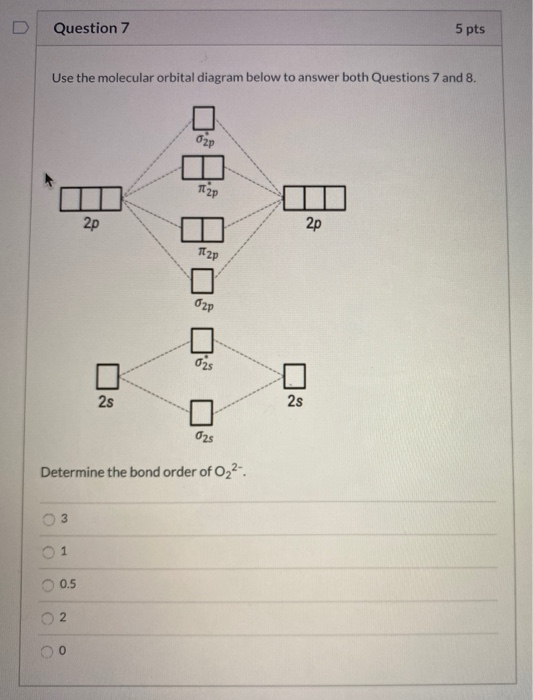
Use the following mo diagram to find the bond order for o2.. Molecular Orbital Theory - Purdue University The bond order in sulfur dioxide, for example, is 1.5 the average of an S-O single bond in one Lewis structure and an S=O double bond in the other. In molecular orbital theory, we calculate bond orders by assuming that two electrons in a bonding molecular orbital contribute one net bond and that two electrons in an antibonding molecular orbital ... Draw MO diagram of CO and calculate its bond order ... Welcome to Sarthaks eConnect: A unique platform where students can interact with teachers/experts/students to get solutions to their queries. Students (upto class 10+2) preparing for All Government Exams, CBSE Board Exam, ICSE Board Exam, State Board Exam, JEE (Mains+Advance) and NEET can ask questions from any subject and get quick answers by subject teachers/ experts/mentors/students. Answered: Use a MO diagram and calculate the bond… | bartleby Predict the bond order and make a sketch of the lowest energy bonding molecular orbital. arrow_forward Draw an MO energy diagram and predict the bond order of Be2 + and Be2- . The increasing order of the bond order of O2, O2-, O2+ and ... Let us calculate the bond order Bond order (B.O) 1/2 × [Number of an electron in antibonding molecular orbitals] - [Number of electrons in bonding molecular orbitals] The higher the order of the bond the greater the pull between the two atoms and the shorter the length of the bond. (1) B.O for O 2 = 1/2 × [10 - 6] B.O for O 2 = 2
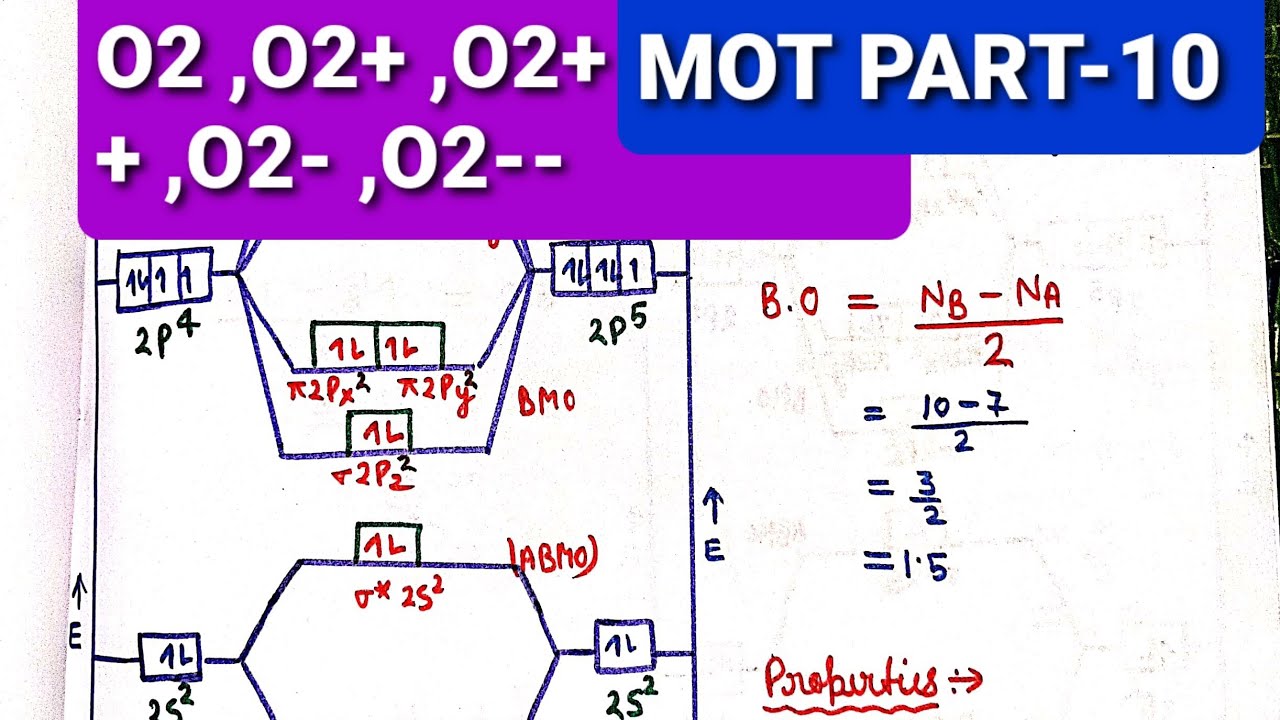
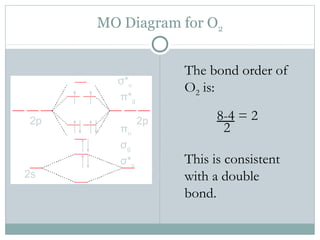
Draw the molecular orbital energy diagram for oxygen ... Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown: (i) Electronic configuration: (ii) Bond order: Here N b = 8; N a = 4 The two oxygen atoms in a molecule of oxygen are united through two covalent ... How to Calculate Bond Order from Molecular Orbital (MO ... 📗 Need help with chemistry? Download 12 Secrets to Acing Chemistry at 💯 If you like my teaching style and are inte... Draw molecular orbital diagram of O2 or N2 with magnetic ... Draw molecular orbital diagram of O 2 or N 2 with magnetic behavior and bond order. Medium Solution Verified by Toppr As it can be seen from the MOT of O 2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b −N a ]/2=[10−6]/2=2. MO Diagrams - GitHub Pages The bond order tells us the average number of bonds between the bonded atoms. In a diatomic molecule such as `O_2`, the bond order simply tells the number of bonds between the two atoms. The bond order can be interpreted from MO diagrams using the following formula: `"Bond Order" = 1/2 [("Bonding "e^-)-("Antibonding " e^-)]`
Answered: a. Using the molecular orbital diagram,… | bartleby a) Use the valence electron molecular orbital diagram for O2 to propose ground state O2 ions that satisfy the following conditions:(i) An O2 ion that is paramagnetic and that has a non-integer bond order.(li) An O2 ion that is paramagnetic and that has an integer bond order.(ili) An O2 ion that is diamagnetic and that has a bond order of 3.Clearly show the charge on your ion, label all valence ... Using the MO diagram of "NO", calculate the bond order ... 4a1 is the σ* 2pz antibonding MO. To obtain the bond order, look at the molecular orbitals formed and decide whether they are bonding or antibonding. BO = 1 2 (bonding e− − antibonding e−) = 1 2 [(2 + 2 + 2 + 2) − (2 + 1)] = 2.5. And this should make sense because NO+ is isoelectronic with CO, which has a bond order of 3. Bond order ofO2 O2+ O2 and O22 is in order A O2 langle ... To solve this question, we need to write the molecular orbital configuration. To find out the bond order from the molecular orbital configuration is: Bond order = 1 2 [ Bonding - antibonding] Complete step by step answer: Let's first draw the MOT of the oxygen molecule. The oxygen molecule O 2 contains the 16 electrons. draw the molecular orbital diagram of n2 also find its ... On the basis of this principle discuss the conditions for obtaining the maximum yield of SO3 in the following reaction. 2SO2(g)+ O2(g)⇌2SO3(g); ∆𝐻= - 42k.cal.(ii) Calculate the pH value of 0.01M CH3 COOH if it is 5% dissociated.
PDF MO Diagrams for Diatomic Molecules Electron Configurations and Bond Orders Just as with atoms, we can write a molecular electron configuration for O2 σ2σ*2σ2π4π*2 We can also calculate the O-O bond order: BO 1 2 # bonding e # anti-bonding e 1 2 8 4 2 LCAO MO theory also predicts (correctly) that O2has two unpaired electrons.
Use the drawing of MO energy diagram for CO to predict the ... The diagram shows how the swing will move forward and then backward after it is initially released. At which point in the diagram is all of the . chemistry. C2H2(g) + 2 H2(g)--> C2H6(g) Bond Bond Energy (kJ/mol) C-C 347 C=C 611 C-H 414 H-H 436 Calculate the value of the C C (triple bond) energy in C2H2 in kJ/mole. ap chemistry
(Get Answer) - Draw molecular orbital diagrams for O2 ... Which has the highest bond order? Which would. Draw molecular orbital diagrams for O2-, O22-, and O2. Which has the highest bond order? Which would be paramagnetic, and which would be diamagnetic? Can you draw good dot structures that correspond to each of these ions or molecules? Mar 22 2022 05:53 AM. Solution.pdf.
What is the bond order of O2+? - Quora To find the bond order of O2+ we can use the concept of Molecular Orbital Theory. In this method we have to count the number of molecules in the Bonding Molecular Orbital and the Anti-Bonding Molecular Orbital. In the 1s shell there are 2 electrons in both BMO and ABMO . Also in the 2s shell there are two electrons in both the BMO and ABMO.
Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration.
Solved re.com/courses/bb687qu7272es/65878 correct Question ... Without 2s-2p mixing With 2s-2p mixing CK :O r 25 20 2s AO MO AO AO MO AO A MO energy levels for O Fa. and Ne B MO energy levels for Ba Cz and Ng 0.5 This problem has been solved! See the answer See the answer See the answer done loading
Draw the molecular orbital diagram of N2 and calculate the ... Draw the molecular orbital diagram of N2 and calculate the bond order. Answer: The bond order shows the number of chemical bonds present between a pair of atoms. The Bond Order Formula can be defined as half of the difference between the number of electrons in bonding orbitals and antibonding orbitals. Bond order formula is given as below.
Solved Use the MO diagram provided below to answer the ... Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (38 ratings) Transcribed image text: Use the MO diagram provided below to answer the following questions: • What is the bond order for C2?_ [Select] • Is C2 ...

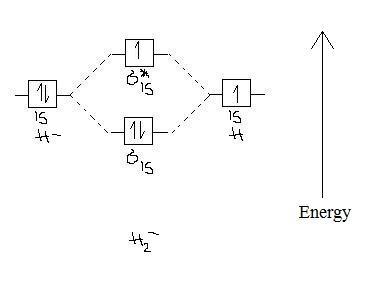




![Expert Answer] Draw the molecular orbital diagram for F2 and ...](https://hi-static.z-dn.net/files/dae/d7baa23a1d4a2ea2c90e0a703e2fd41d.jpg)


















0 Response to "42 use the following mo diagram to find the bond order for o2."
Post a Comment